Article Text
Abstract
Background Primary percutaneous coronary intervention (PCI) is a proven therapy for acute ST-segment elevation myocardial infarction. However, outcomes associated with primary PCI may differ depending on time of day.
Methods and results Using the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease, a clinical data-collection initiative capturing all cardiac catheterisation patients in Alberta, Canada, the authors described and compared crude and risk-adjusted survival for ST-segment elevation myocardial infarction patients undergoing primary PCI after-hours versus regular working hours. From 1 January 1999 to 31 March 2006, 1664 primary PCI procedures were performed (54.4% after-hours). Mortalities at 30 days were 3.6% for regular hours procedures and 5.0% for after-hours procedures (p=0.16). 1-year mortalities were 6.2% and 7.3% in the regular hours and after-hours groups, respectively (p=0.35). After adjusting for baseline risk factor differences, HRs for after-hours mortality were 1.26 (95% CI 0.78 to 2.02) for survival to 30 days and 1.08 (0.73 to 1.59) for survival to 1 year. A meta-analysis of our after-hours HR point estimate with other published risk estimates for after hours primary PCI outcomes yielded an RR of 1.23 (1.00 to 1.51) for shorter-term outcomes.
Conclusions After-hours primary PCI was not associated with a statistically significant increase in mortality. However, a meta-analysis of this study with other published after-hours outcome studies yields an RR that leaves some questions about unexplored factors that may influence after-hours primary PCI care.
- Infarction
- mortality
- angioplasty
- stents
- adverse event
- morbidity and mortality
- patient outcomes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
Statistics from Altmetric.com
Introduction
Primary percutaneous coronary intervention (PCI) is superior to thrombolysis for acute ST-segment elevation myocardial infarction (STEMI).1 An important factor affecting outcome in primary PCI is delays to treatment,2–4 a particular concern after regular working hours, when facilities must be activated and staff brought in from home. Some institutions are therefore concerned that favourable outcomes may be difficult to achieve for patients presenting after hours. In a period of constrained resources, this would not lead to endorsement of routine after-hours procedures, and may in fact lead to scrutiny of how medical facilities operate at night, including more widespread adoption of night shifts.
Recent attention has also been directed towards other causes of adverse patient outcomes occurring after hours, mostly related to the effects of sleep deprivation and fatigue on healthcare provider performance, process of care and medical error.5–13 While none of these data are specifically related to cardiac care, one can postulate that these important factors might be at play in the provision of primary PCI.
We have developed a large, population-based, clinical registry capturing all patients undergoing cardiac catheterisation and revascularisation in Alberta, Canada, which provides a unique opportunity to evaluate outcomes in unselected patients. We sought to describe and compare crude and risk-adjusted survival for patients undergoing primary PCI for acute STEMI after-hours to those whose procedures occurred during regular working hours.
Methods
Data sources
The Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) is a clinical data-collection initiative capturing consecutive patients undergoing cardiac catheterisation in Alberta, Canada (population 3 290 350) since 1995.14 APPROACH contains detailed information including patients' age, sex, ejection fraction and multiple comorbidities as outlined in table 1. It tracks therapeutic interventions (previous thrombolytic therapy, revascularisation procedures). Coronary anatomy and procedural details are also recorded. Following data entry by catheterisation laboratory staff, an enhancement procedure verifies patient comorbidities and ensures that there are no missing data fields.15 Follow-up mortality for all patients is ascertained through semiannual linkage to the Alberta Bureau of Vital Statistics. Three hospitals in two large cities (Edmonton and Calgary) provide the only revascularisation services in Alberta, and primary PCI is the preferred treatment strategy for STEMI. APPROACH and this protocol were approved by the Institutional Review boards of the University of Alberta and the University of Calgary.
Baseline Characteristics
The study population for this analysis consisted of STEMI patients undergoing primary PCI. Rescue PCI patients and those requiring hospital transfer were excluded. Door-to-balloon times for all patients were obtained through linkages to emergency room administrative data, and to the Calgary STEMI quality improvement data registry, which has prospectively collected time-interval data since 2004.
Timing of PCI procedures
Data in APPROACH are entered in real-time, with a database ‘clock’ for regular working hours (weekdays 0700–1800) or after-hours (weeknights 1800–0700, weekends and holidays). In order to measure outcomes using currently available technology and adjunctive therapy, we limited our assessment to those patients undergoing primary PCI from 1 January 1999 to 31 March 2006.
Outcome measures
Our primary goal was to determine whether after-hours procedures were associated with higher crude and adjusted mortalities at 30 days. A secondary analysis assessed survival to 1 year, though we recognise a priori that many factors can intervene over this period to potentially dilute any influence of the timing of PCI on outcomes.
Statistical analysis
Patient characteristics were compared using χ2 tests. Kaplan–Meier plots and logrank tests were used to determine and compare crude mortalities. Multivariable Cox proportional hazards models were then used to adjust for the effects of baseline risk factors on group survival. The proportion hazards assumption was tested.16 The variables used for risk-adjustment analysis in these models are the baseline variables recorded in APPROACH (presented in table 1).14
Additional analysis including door-to-balloon time
The distributions of door-to-balloon times were described using simple box plots. These times were then entered as independent variables in the above-mentioned multivariable Cox proportional hazards models that included all of the baseline clinical variables, to determine whether adjustment for door-to-balloon times changed the point estimates of our adjusted HRs and were therefore a mediating factor of any potential associations of time of day with mortality. An additional sensitivity analysis using door-to-balloon time ≤90 min or >90 min (according to current guidelines for optimal performance of primary PCI) was performed, with door-to-balloon time first assessed as a potential confounding variable (through inclusion in the multivariable models), and then as an effect modifier (through stratification on door-to-balloon time).
We performed a meta-analysis of our study's RR for after-hours PCI along with other published studies, to place our findings in the context of what is already known about this important question. A detailed literature search identified all published manuscripts on this topic. The search strategy and study selection procedures are available from the authors upon request. Because of heterogeneity noted in the relative risks across studies (τ2 0.051, p=0.02), a random effects model was chosen for pooling of results across studies.
Statistical analyses were performed using SAS Version 8.1. The meta-analysis was performed using Stata Version 8.
Results
Patient characteristics
From 1 January 1999 to 31 March 2006, 1664 patients underwent primary PCI for acute MI in Alberta. Of these, 54.4% occurred after hours. Table 1 shows the baseline characteristics of regular working hours and after-hours cases. There were no significant differences between the groups in terms of cardiac risk factors, comorbidities, ejection fraction, extent of coronary disease or culprit vessel, with the exception of a higher use of intra-aortic balloon counterpulsation devices (alone or in combination with inotropes) during working hours, and higher use of inotropes alone after-hours.
Crude and adjusted outcomes
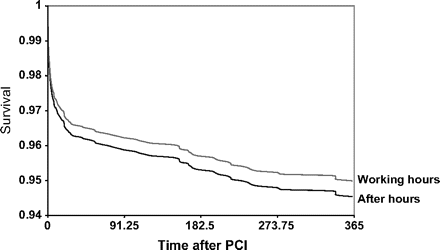
Mortalities at 30 days were 3.6% in the working hours group and 5.0% in the after-hours group (p=0.16). By 1 year, mortalities were 6.2% and 7.3% in the working hours and after-hours groups, respectively (p=0.35). Figure 1 shows Kaplan–Meier survival curves extending to 1 year of follow-up. After-hours patients do appear to have a poorer survival over time.
Kaplan–Meier survival curves to 1 year for primary percutaneous coronary intervention (PCI) performed after hours and during regular working hours.
Table 2 shows the HRs and 95% CIs of 1.34 (95% CI 0.85 to 2.12) for after-hours cases relative to working hours cases for survival extending to 30 days (our primary study outcome) and 1.18 (95% CI 0.81 to 1.72) for survival extending to 1 year. After adjusting for the variables in table 1, HRs (HR-1) changed slightly to 1.26 (95% CI 0.78 to 2.02) for survival to 30 days and 1.08 (95% CI 0.73 to 1.59) for survival to 1 year.
Crude and Adjusted Hazard Ratio for survival for after-hours relative to regular hours primary PCI
Analysis controlling for door-to-balloon times
The median door-to-balloon time was 72.0 min in the working hours group and 80.0 min in the after-hours group (p=0.007), as demonstrated by the box plots in figure 2.
Boxplots illustrating door-to-balloon times for primary percutaneous coronary intervention performed after hours and during regular working hours. The median door-to-balloon time is indicated. The boundaries of the box plots refer to the 25th and 75th percentiles, with the whisker bars representing the 5th and 95th percentiles.
Table 2 also presents the HRs for survival associated with after-hours procedures, further adjusted for door-to-balloon times (HR-2, 1.23 (95% CI 0.77 to 1.99)). The sequential analysis for survival to 1 year also revealed a minimal effect of this additional adjustment. The full Cox regression model can be found in appendix 2. An additional sensitivity analysis performed using the door-to-balloon time cutpoints of ≤90 min and >90 min, treated as a confounding variable through inclusion in the multivariable models, yielded HRs that were essentially the same (30-day survival HR 1.23 (95% CI 0.77 to 1.99)). When considered as an effect modifier in stratified analyses, we found a stronger association for those with longer door-to-balloon times (≤90 min HR 1.23 (95% CI 0.63 to 2.42); >90 min HR 1.53 (95% CI 0.76 to 3.09)).
Meta-analysis
To present our study result more explicitly in the context of the existing literature, we performed a meta-analysis of studies examining outcomes in after-hours primary PCI (figure 3). The studies ranged from single centre experiences to large registries, and one clinical trial of PCI strategies (CADILLAC), conducted from 1994 to 2006. Several excluded cardiogenic shock, rescue PCI or transfer patients.17–21 A tabulated description of these studies is presented in appendix 1. Unadjusted risk ratios ranged from 0.61 to 6.54, with an overall random-effect pooled estimate of RR of 1.23 (95% CI 1.00 to 1.52). This pooled result across 12 studies, including ours, does suggest that there may still be a need to continue exploring the possibility of an association between after-hours procedures and poorer outcomes.
Meta-analysis of studies examining outcomes of primary percutaneous coronary intervention performed after hours and during regular working hours.
Discussion
Our study adds to a growing body of literature on after-hours medical care. In an unselected patient population, outcomes for after-hours PCI cases did not differ significantly from those of working-hours cases. However, the point estimate from our study suggesting a 23% increased risk for adverse events early after PCI needs to be taken in the context of other studies, some of which have shown poorer outcomes in after-hours primary PCI. Further, the 23% increase seen in this study and our meta-analysis of prior studies is hardly negligible in that it is of similar magnitude to the benefits associated with beta-blocker and thrombolytic therapy for STEMI.22 23
Interest in after-hours care has heightened with increased international focus on patient safety. Such issues have received considerable attention in relation to studies that have demonstrated increased mortality in patients with severe medical conditions admitted on weekends, as a direct result of delayed care.24 Kostis and colleagues also found that weekend admissions for patients with MI were associated with a higher mortality.25
With primary PCI, concerns about outcomes are most important after-hours cases where the need to bring cardiac catheterisation laboratory staff from home may result in significant treatment delays. Previous investigations show conflicting results. Garot and colleagues assessed 288 primary PCI patients and found similar door-to-balloon times and no differences in in-hospital outcome.17 However, this study was conducted in a French centre which activates the cardiac catheterisation laboratory from the ambulance and is staffed after-hours by in-house nurses. It is difficult to apply the findings of this study to areas which lack these policies. Zahn et al examined the outcomes of 378 patients treated during regular working hours and 113 patients treated after-hours, where mortality was lower (5.3% vs 8.7%) in the after-hours group.26 However, eight facilities participated during working hours but fewer at night, raising the possibility of selection bias in after-hours cases. Data from the 2082 patients enrolled in the larger randomised CADILLAC primary PCI trial found that patients who presented after hours had similar 30-day and 1-year mortalities to those presenting during working hours.18 In contrast, in 1702 consecutive primary PCI cases, Henriques et al found that patients treated off-hours had a higher incidence of failed PCI and worse clinical outcomes, including increased 30-day mortality.27
We noted a lack of effect of controlling for door-to-balloon times on our point estimate of RR, even when using the accepted clinical cutpoint of ≤90 min as a confounding variable. When treated as an effect modifier in stratified analyses, we found a stronger association of hazard for those with longer door-to-balloon times (>90 min), suggesting that the impact of the after-hours construct is even greater when treatment is delayed. These findings, and the potential signal of harm suggested by the meta-analysis presented here, require us to consider other possible contributing explanations for increased mortality in after-hours patients. One possibility is that physician fatigue could influence procedural performance, well represented in the anaesthesia literature.6–8 In addition, in a study of the effect of heavy night call in residents, Arnedt et al found that postcall impairment was at least equivalent to the ingestion of 3–4 standard alcoholic drinks.9 Other investigators have found that manual dexterity and surgical skills may be specifically vulnerable to sleep deprivation.10–12 28
Staffing levels also tend to be lower on weekends and holidays than during working hours, despite often increased patient acuity, and are a potential contributor to suboptimal patient safety at such times.29–31
Another important concern relevant to our cohort relates to the fact that all revascularisation procedures in Alberta are performed in academic tertiary care centres, and overnight care outside the cardiac catheterisation laboratory is generally provided by junior housestaff. Serious medical errors and pronounced increases in after-hours mortality have both been demonstrated in major teaching hospitals, whereas after-hours admissions to tertiary care intensive care units with on-site attending physicians are not associated with increased mortality.13 31–33 Thus, the combination of relatively inexperienced housestaff, low staffing and fatigue among providers may be responsible for some of the suggestion of increased hazard associated with after-hours primary PCI.
There are limitations to this study. Like other investigators studying acute MI care, we do not have any data regarding symptom onset-to-balloon time, which is difficult to characterise at night, as the perceived time of symptom onset may not reliably reflect actual ischaemic time, and patients who are at home when symptoms occur may be less likely to promptly seek medical attention. All PCI procedures were performed by experienced operators at high-volume academic centres, so our results may not be generalisable to patients in other settings, or to hospitals that do not rely upon trainees for major provision of after-hours care. Finally, our thoughts as to the other potential influences on after-hours outcomes remain speculative, as none of the above-mentioned studies are specific to cardiology.
The above notwithstanding, our findings do not support abandoning after-hours primary PCI in favour of thrombolysis. Given that the major studies of primary PCI versus thrombolysis would have included at least some after-hours patients in both treatment arms, it is unlikely that the benefit of primary PCI would be entirely negated after-hours. In addition, potential factors influencing outcomes after-hours could also apply to patients receiving thrombolysis.
In conclusion, our study findings suggest that primary PCI can be performed outside a clinical trial with acceptable short- and long-term mortalities, during working hours and after-hours. However, our findings taken in the context of other after-hours primary PCI studies, with an almost 25% increase in the risk for short-term mortality, do not provide complete reassurance; nor do they indicate complete equivalency of outcomes to working-hours procedures. This summary finding remains a concern and may be related to previously unexplored areas in after-hours care. Patient satisfaction will also need to be considered. Further research is thus still required to determine whether processes and quality of care are influenced by understudied areas such as fatigue, staffing levels, physician experience or other factors.
Acknowledgments
We would like to thank R Boone, for his thoughtful comments. We appreciate the assistance of the Calgary Health Region and the Capital Health Authority in supporting data entry by cardiac catheterisation laboratory personnel.
Appendix 1
| Study | Overall N | Location | Inclusion criteria | Exclusion criteria | Working hours mortality | After-hours mortality | RR (95% CI) |
| Dominguez-Rodriguez et al34 | 90 | Spain, 2003 | Consecutive primary PCI, single centre | None identified | 1/51 (1.9%) in hospital | 5/39 (12.8%) | 6.54 (0.80 to 53.72) |
| Assali et al20 | 273 | Israel, 2001–2004 | Consecutive primary PCI, single centre | Cardiogenic shock | 2/160 (1.25%) in hospital 5/160 (3.1%) at 30 days | 7/113 (6.2%) 11/113 (9.7%) | 4.96 (1.05 to 23.42) |
| Ortolani et al21 | 985 | Italy, 2003–2005 | Consecutive primary PCI, single centre | Rescue PCI, in-hospital ST-segment elevation myocardial infarction | 29/382 (7.6%) in hospital | 49/603 (8.1%) | 1.06 (0.69 to 1.63) |
| Saleem et al35 | 1050 | USA, 1998–2002 | Consecutive primary PCI, single centre | 21/656 (3.2%) in hospital | 23/394 (5.8%) | 1.82 (1.02 to 3.25) | |
| Sadeghi et al18 | 2036 | International | CADILLAC randomized controlled trial, all sites 24/7 primary PCI | Shock, bleeding, renal insufficiency | 17/1047 (1.6%) at 30 days | 24/989 (2.4%) | 1.49 (0.81 to 2.76) |
| Henriques et al27 | 1702 | Netherlands, 1994–2000 | Consecutive primary PCI, within 6 h, single centre | Symptom onset >6 h | 17/909 (1.0%) at 30 days | 33/793 (4.2%) | 1.72 (1.18 to 2.51) |
| Magid et al19 | 33647 | USA, 1999–2002 | NRMI registry, PCI at 421 centres | Transfer patients | 728/15419 (4.7%) | 859/18228 (4.7%) in hospital | 1.0 (0.91 to 1.10) |
| Slonka et al36 | 1778 | Poland, 1998–2003 | Consecutive primary PCI, single centre, working hours defined as 0800–1500 | 33/482 (6.8%) in hospital | 80/1296 (6.2%) | 0.90 (0.61 to 1.33) | |
| Srimachochota37 | 256 | Thailand, 1999–2003 | Consecutive primary PCI, single centre | 11/107 (10.3%) in hospital | 16/149 (10.7%) | 1.04 (0.51 to 2.16) | |
| Zahn et al26 | 491 | Germany, 1994–1997 | MITRA registry, consecutive primary PCI at eight centres during the day and three centres at night (concern for selection bias—23% of patients done after-hours) | 33/378 (8.7%) in hospital | 6/113 (5.3%) | 0.61 (0.26 to 1.41) | |
| Garot et al17 | 288 | France | Consecutive primary PCI, <6 h after symptom onset, cath lab activated by cath lab staffed after hours by CCU nurses | Shock | 6/113 (5.3%) | 12/175 (6.9%) | 1.29 (0.50 to 3.34) |
| Graham | 2043 | Alberta, 1999–2006 | Consecutive primary PCI, three centres | Transfer patients | 32/896 (3.6%) | 57/1147 (5.0%) | 1.39 |
PCI, percutaneous coronary intervention.
Appendix 2
Full Cox regression model (30 days and 1 year)
30-day
| Model fit statistics | ||
| Criterion | Without covariates | With covariates |
| –2 log L | 1153.810 | 1050.941 |
| AIC | 1153.810 | 1090.941 |
| SBC | 1153.810 | 1138.075 |
| Testing global null hypothesis: beta=0 | |||
| Test | χ2 | df | Pr>χ2 |
| Likelihood ratio | 102.8685 | 20 | <0.0001 |
| Score | 148.7902 | 20 | <0.0001 |
| Wald | 120.9245 | 20 | <0.0001 |
| Analysis of maximum likelihood estimates | |||||||
| Parameter | df | Parameter estimate | SE | χ2 | Pr>χ2 | HR | 95% CI |
| After hour | 1 | 0.22990 | 0.24166 | 0.9050 | 0.3414 | 1.26 | 0.78 to 2.02 |
| Age | 1 | 0.41968 | 0.26344 | 2.5379 | 0.1111 | 1.52 | 0.91 to 2.55 |
| Sex | 1 | −0.62751 | 0.24654 | 6.4781 | 0.0109 | 0.53 | 0.33 to 0.87 |
| COPD | 1 | −0.05550 | 0.39203 | 0.0200 | 0.8874 | 0.95 | 0.44 to 2.04 |
| CEVD | 1 | 0.51473 | 0.38828 | 1.7574 | 0.1849 | 1.67 | 0.78 to 3.58 |
| Creat | 1 | 1.20996 | 0.38643 | 9.8038 | 0.0017 | 3.35 | 1.57 to 7.15 |
| Diabetes | 1 | 0.69557 | 0.26984 | 6.6446 | 0.0099 | 2.01 | 1.18 to 3.40 |
| Dialysis | 1 | −0.21161 | 0.71513 | 0.0876 | 0.7673 | 0.81 | 0.20 to 3.29 |
| HTN | 1 | −0.64629 | 0.25774 | 6.2878 | 0.0122 | 0.52 | 0.32 to 0.87 |
| Lipid | 1 | −1.04936 | 0.29260 | 12.8618 | 0.0003 | 0.35 | 0.20 to 0.62 |
| Liver/GI | 1 | 0.25309 | 0.64277 | 0.1550 | 0.6938 | 1.29 | 0.37 to 4.54 |
| Malignancy | 1 | −1.02538 | 1.02202 | 1.0066 | 0.3157 | 0.36 | 0.05 to 2.66 |
| Old MI | 1 | −0.15068 | 0.37330 | 0.1629 | 0.6865 | 0.86 | 0.41 to 1.79 |
| Lytic | 1 | −0.12468 | 0.72190 | 0.0298 | 0.8629 | 0.88 | 0.21 to 3.63 |
| PVD | 1 | 0.87345 | 0.36897 | 5.6040 | 0.0179 | 2.40 | 1.16 to 4.94 |
| Ef 35 | 1 | −0.51292 | 0.28934 | 3.1425 | 0.0763 | 0.60 | 0.34 to 1.06 |
| Ef 20 | 1 | 0.09989 | 0.40171 | 0.0618 | 0.8036 | 1.11 | 0.50 to 2.43 |
| Ef under20 | 1 | 1.08157 | 1.06411 | 1.0331 | 0.3094 | 2.95 | 0.37 to 23.74 |
| d1 | 1 | 0.83690 | 0.26202 | 10.2018 | 0.0014 | 2.31 | 1.38 to 3.86 |
| d2 | 1 | 1.39078 | 0.40562 | 11.7563 | 0.0006 | 4.02 | 1.81 to 8.90 |
1-year
| Model fit statistics | ||
| Criterion | Without covariates | With covariates |
| –2 log L | 1668.884 | 1528.640 |
| AIC | 1668.884 | 1568.640 |
| SBC | 1668.884 | 1623.187 |
| Testing global null hypothesis: beta=0 | |||
| Test | χ2 | df | Pr>χ2 |
| Likelihood ratio | 140.2445 | 20 | <0.0001 |
| Score | 210.1061 | 20 | <0.0001 |
| Wald | 166.3952 | 20 | <0.0001 |
| Analysis of maximum likelihood estimates | |||||||
| Parameter | df | Parameter estimate | SE | χ2 | Pr>χ2 | HR | 95% CI |
| After hour | 1 | 0.07764 | 0.19810 | 0.1536 | 0.6951 | 1.08 | 0.73 to 1.59 |
| Age | 1 | 0.72647 | 0.21061 | 11.8978 | 0.0006 | 2.07 | 1.37 to 3.12 |
| Sex | 1 | −0.49431 | 0.20938 | 5.5735 | 0.0182 | 0.61 | 0.41 to 0.92 |
| COPD | 1 | −0.40181 | 0.34744 | 1.3375 | 0.2475 | 0.67 | 0.34 to 1.32 |
| CEVD | 1 | 0.61525 | 0.31582 | 3.7951 | 0.0514 | 1.85 | 1.00 to 3.44 |
| Creat | 1 | 1.32714 | 0.31433 | 17.8261 | <0.0001 | 3.77 | 2.04 to 6.98 |
| Diabetes | 1 | 0.81857 | 0.21788 | 14.1147 | 0.0002 | 2.27 | 1.48 to 3.48 |
| Dialysis | 1 | −0.49067 | 0.61541 | 0.6357 | 0.4253 | 0.61 | 0.18 to 2.05 |
| HTN | 1 | −0.50147 | 0.21114 | 5.6409 | 0.0175 | 0.61 | 0.40 to 0.92 |
| Lipid | 1 | −0.67676 | 0.22209 | 9.2852 | 0.0023 | 0.51 | 0.33 to 0.79 |
| Liver/GI | 1 | 0.28563 | 0.49074 | 0.3388 | 0.5605 | 1.33 | 0.51 to 3.48 |
| Malignancy | 1 | 0.43663 | 0.44218 | 0.9750 | 0.3234 | 1.55 | 0.65 to 3.68 |
| Old MI | 1 | 0.05295 | 0.28561 | 0.0344 | 0.8529 | 1.05 | 0.60 to 1.85 |
| Lytic | 1 | −0.10150 | 0.59082 | 0.0295 | 0.8636 | 0.90 | 0.28 to 2.89 |
| PVD | 1 | 0.82531 | 0.31353 | 6.9289 | 0.0085 | 2.28 | 1.24 to 4.22 |
| Ef 35 | 1 | −0.58321 | 0.24534 | 5.6509 | 0.0174 | 0.56 | 0.35 to 0.90 |
| Ef 20 | 1 | 0.08877 | 0.33432 | 0.0705 | 0.7906 | 1.09 | 0.57 to 2.10 |
| Ef under20 | 1 | 0.37509 | 1.05195 | 0.1271 | 0.7214 | 1.46 | 0.19 to 11.44 |
| d1 | 1 | 0.78256 | 0.21761 | 12.9322 | 0.0003 | 2.19 | 1.43 to 3.35 |
| d2 | 1 | 1.44161 | 0.32632 | 19.5168 | <0.0001 | 4.23 | 2.23 to 8.01 |
COPD – chronic pulmonary disease
CEVD – cerebrovascular disease
HTN – hypertension
MI – myocardial infarction
Lytic – thrombolytic therapy
PVD – peripheral vascular disease
Ef – Ejection Fraction
References
Footnotes
APPROACH Clinical Steering Committee: Edmonton—R Tsuyuki (chair), M Graham, A Koshal; Calgary—M Curtis, WA Ghali, ML Knudtson, A Maitland, L Brent Mitchell and M Traboulsi.
Funding The research and creation of this paper were supported by a grant from the Canadian Institutes of Health Research (CIHR). MLK receives partial support from the Libin Trust Fund. WAG is a Senior Health Scholar of the Alberta Heritage Foundation for Medical Research and also supported by a government of Canada Research Chair in Health Services Research and by a Health Scholar Award from the Alberta Heritage Foundation for Medical Research, Edmonton, Alberta. APPROACH was funded in 1995 by the Weston Foundation, with ongoing support from the Canadian Cardiovascular Outcomes Research Team (CCORT), a CIHR Team Grant and the Province-Wide Services Committee of Alberta Health and Wellness. The initiative has also received unrestricted support from Merck Frosst Canada, Monsanto Canada—Searle, Eli Lilly Canada, Guidant Corporation, Boston Scientific, Hoffmann-La Roche and Johnson & Johnson Inc—Cordis.
Competing interests None.
Ethics approval Ethics approval was provided by the University of Calgary and University of Alberta.
Provenance and peer review Not commissioned; externally peer reviewed.