Abstract
Background:
Actinic keratosis lesions occur frequently on sun-exposed skin of Caucasians. They become more prevalent with advancing age and are important in identifying the risk factor of those people possibly predisposed to invasive squamous cell carcinoma. Topical therapies are useful alternatives to cryotherapy for treating diffuse actinic damage and a number of preparations have been developed for treating actinic keratosis.
Objectives:
A cumulative meta-analysis was performed to determine the efficacy of imiquimod 5% cream, which presents a new alternative topical therapy for actinic keratosis, and to compare it to 5-fluorouracil for the treatment of actinic keratosis lesions of the face and scalp.
Methods:
We searched MEDLINE (1966 to October 2004) for relevant studies evaluating the efficacy of actinic keratosis topical agents imiquimod and 5-fluorouracil (0.5%, 1%, and 5%). Studies included in this meta-analysis required a dosage regimen that was not significantly different from that approved by Health Canada and the U.S. FDA. Studies also required a well-defined treatment duration and followup period, with the primary efficacy variable being the complete (100%) clearance of all actinic keratosis lesions defined as the proportion of patients at followup with no clinically visible lesions in the treatment area. To determine the average efficacy rate for both drugs, the data from each study were combined for that drug.
Results:
Ten studies were included in the analysis. The average efficacy rate for each drug (with 95% confidence interval) was 5-fluorouracil, 52 ± 18% (n = 6 studies, 145 subjects) and imiquimod, 70 ± 12% (n = 4 studies, 393 subjects).
Conclusions:
The results of this meta-analysis show that both imiquimod and 5-fluorouracil are effective methods for the treatment of actinic keratosis and provide a useful alternative to cryotherapy. However, this analysis suggests that imiquimod may have higher efficacy than 5-fluorouracil for actinic keratosis lesions located on the face and scalp and therefore provides another option to dermatologists.
Antécédents:
L’apparition de lésions de kératose actinique sur la peau de sujets blancs est fréquente après l’exposition au soleil. Avec l’âge, ces lésions qui deviennent plus. répandues sont importantes dans 1’identification des facteurs de risque chez les sujets qui seraient prédisposes à être victimes d’une invasion de careinomes squameux. Les thérapies topiques sont des alternatives utiles à la cryotherapie dans le traitement des lésions actiniques diffuses. Un nombre de produits formulés ont êtê mis au point pour le traitement de la kératose actinique.
Objectifs:
Une méta-analyse cumulative a été effectuée en vue de déterminer 1’efficacité de la crème d’imiquimod à 5%, qui représente une nouvelle therapie topique alternative de la kératose actinique, et de la comparer à 5-fluorouracil dans le traitement des lésions de kératose actinique sur le visage et le cuir chevelu.
Méthodes:
Nous avons cherché dans MEDLINE (de 1966 jusqu’à octobre 2004) les études pertinentes qui évaluent l’efficacité des agents topiques imiquimod et 5-fluorouracil (0.5%, 1%, et 5%) dans le traitement des kératoses actiniques. Les études dont la présente méta-analyse tient compte suivent un dosage peu différent du dosage approuvé par Sante Canada et la FDA aux États-Unis. Les études doivent également comprendre une durée de traitement bien définie et une période de suivi. La principale variable d’efficacité est 1’épuration totale de la peau (100%) de toutes les lésions actiniques, définie comme la proportion de patients qui, au suivi, ne présentait aucune trace de lésions dans la zone traitée. Afin de déterminer le taux d’efficacité moyen de chacun des deux médicaments, les données de chaque étude ont été combinées.
Résultats:
Dix études ont été comprises dans 1’analyse. Le taux d’efficacite moyen de chaque médicament (avec un intervalle de confiance de 95%) est:5-fluorouracil, 52 ± 18% ( n = 6 études, 145 sujets) et imiquimod, 70 ± 12% (n = 4 etudes, 393 sujets).
Conclusion:
Les résultats de cette méta-analyse montrent que l’imiquimod et 5-fluorouracil sont tous deux efficaces dans le traitement des kératoses actiniques et représentent des alternatives utiles à la cryothérapie. Toutefois, cette étude suggère que l’imiquimod serait plus efficace que 5-fluorouracil dans le traitement des lésions de kératose actinique qui se trouvent sur le visage et le cuir chevelu, et représenterait done une autre option de traitement pour les dermatologues.
Similar content being viewed by others
Actinic keratosis (AK) affects approximately 1 in 6 North Americans during their lifetime with over 1 million new cases reported annually. 1 , 2 Actinic keratoses are scaly lesions, precursorous to squamous cell carcinoma, that occur on areas of sun exposure, predominantly on the face, bald scalp, hands, and forearms.3,4 Current treatments include cryotherapy, excision, and topical therapies, or a combination of these therapeutic modalities.5–8 Topical 5-fluorouracil (5-FU) is a well-known nonsurgical treatment for AK and is available in 0.5%, 1% and 5% formulations.9–15 As a schemotherapeutic agent, fluorouracil essentially destroys AK by interfering with DNA and RNA synthesis by blocking the methylation reaction of deoxyuridylic acid to thymidylic acid. This causes thymine deficiency, particularly in cells mat are growing quickly and taking up fluorouracil at a more rapid rate, leading to unbalanced growth and death of these cells.16 Recently, imiquimod (5%) cream (AldaraTM) received Canadian and U.S. Food and Drug Administration (U.S. FDA) approval for the treatment of AK. The therapeutic effect of imiquimod against AK lesions is more physiologic in the way that it mimicks the body’s own natural immune response to cells expressing tumoral antigens.
Imiquimod stimulates the innate immunity by binding to toll-like receptor (TLR) 7 and inducing synthesis and release of cytokines such as interferon-α (INF-α), interleukin-12 (IL-12), and tumor necrosis factor-α (TNF-α).17–20 It also activates Langerhans cells to process the antigens and migrate to the regional lymph nodes. The resulting influx of macrophages and activated cytotoxic T lymphocytes leads to the apoptosis of AK cells.
Since imiquimod presents a new alternative topical therapy for AK, the purpose of this study was to determine the efficacy of imiquimod 5% cream and to compare it to 5-fluorouracil (5-FU) for treatment of AKs of the face and scalp, by performing a cumulative meta-analysis of studies for each drug.
Materials and Methods
Inclusion and Exclusion Criteria
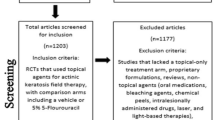
The studies included in the meta-analysis had evaluated the efficacy of imiquimod (5%) and 5-fluorouracil (0.5%, 1%, and 5%) topical creams for treating actinic keratoses of the face and scalp. A MEDLINE search (1966 to October 2004) was performed for relevant studies. To be included in the meta-analysis, studies had to meet specific criteria and contain data that could be readily extracted. Treatment areas included only the face and/or scalp. Drug regimens were as follows: 0.5% 5-FU once daily, 2–4 weeks; 1% 5-, FU twice daily, 2–6 weeks; 5% 5-FU twice daily, 2–4 weeks; and imiquimod 2–3 times weekly, up to 16 weeks. The followup period for 5-FU ranged between 4 weeks and 11 and months post-treatment, while data for imiquimod were analyzed between 2 and 8 weeks post-treatment. The primary efficacy variable was the complete clearance of all AK lesions defined as the proportion of patients at followup with no clinically visible AKs in the treatment area.
Studies were excluded when the above inclusion criteria were not met, when treatment efficacy was not assessed for AKs of the face or scalp, when the topical fluorouracil was not a 0.5%, 1%, or 5% cream, when the treatment regimen was significantly different from the one approved by Health Canada or the U.S. FDA (i.e., pulse or cycling regimen, etc.), when the study report was not in English, or when the data were duplicated from a previous publication.
Statistics
The method designed by DerSimonian and Laird21 and later modified by Velanovich22 for single-group analysis was used to combine the efficacy data for each drug. This method uses a sample-size weighted average for each efficacy rate. A 95% confidence interval (CI) (mean ± 1.96 standard error [SE]) was calculated from the derived value for the SE.
Results
Ten studies were included in this meta-analysis.9,13,15,23–29 Seventeen studies were excluded since they did not meet the inclusion criteria for various reasons (Table I).30–46
Meta-analysis of 5-Fluorouracil
The efficacy rates found for treating actinic keratoses of the face and/or anterior bald scalp with topical fluorouracil are summarized in Table II.9,13,15,23–25,27 The primary efficacy variable was the complete (100%) clearance rate. Complete clearance was defined as the proportion of patients with no clinically visible AK lesions in the treatment are at the post-treatment followup visit. Patient response was quite variable among these studies. Breza et al.[23] performed a split-face trial using 1% 5-FU alone and 1% 5-FU in combination with topical triamcinolone acetonide to determine its effectiveness in suppressing the unpleasant, painful inflammatory reaction caused by topical 5-FU when used as a therapy for AK. At one month post-treatment, 70% of the subjects had complete clearance of lesions.
In the study by Dillaha et al.9 Various concentrations of 5-FU were applied to the entire face, neck, arms, or hands to evaluate the optimal concentration needed for treating AK lesions and to obtain data regarding systemic absorption. Only data pertaining to AKs of the head were extracted for this meta-analysis. At the 11-month follow-up appointment, complete clearance was seen in 0% and 86.4% of the 1% 5-FU and 5% 5-FU groups, respectively.
In their split-face study, Lawrence et al.25 reported complete clearance of facial AKs in 6.7% of subjects one month following treatment with 5% 5-FU, while the same concentration of 5-FU resulted in complete clearance of lesions in 100% of subjects in the comparison study by Shuttleworth and Marks,27 six weeks following two weeks of treatment.
The 0.5% 5-FU phase III studies showed 57.8%13 and 47.5%15 of patients treated for AKs on the face and frontal scalp for four weeks had complete clearance at four weeks post-treatment followup for a combined efficacy rate of 52.7%.
The variability of efficacy rates for these studies demonstrates the need for meta-analysis. The meta-analytical average for the patient response rate following topical fluorouracil treatment was 52.2 ± 18% (mean ± 95% CI).
Meta-analysis of Imiquimod
Table III summarizes the efficacy rates for treating AKs with topical imiquimod cream.24,26,28,29 The primary efficacy variable was complete clearance rate with complete clearance defined as the proportion of subjects at the post-treatment visit with no clinically visible AK lesions in the treatment area. The number of lesions cleared included both the baseline lesions and any subclinical AK lesions that appeared during therapy.
Stockfleth et al.28,29 reported two studies that met the inclusion criteria for this meta-analysis; although the initial dosing regimen in both studies was 3 times a week, it was then decreased to the approved 2-times-a-week regimen and was used continuously for no longer than 16 weeks, as per the labeling. In their initial case study,28 complete clearance of lesions was reported in each of the six patients (100%). Complete clearance in 84% of the 25 subjects treated with imiquimod cream was reported in the second study.29
The phase III trials reported by Lebwohl et al.26 resulted in 97/215 subjects having complete (100%) clearance of AK lesions at the two-month followup visit.
Szeimies et al.24 reported a phase III trial in which imiquimod was administered 3 times a week for 16 weeks on the face and scalp resulting in 57.1% of patients having complete clinical and histologic clearance.
For the studies using imiquimod to treat AKs, the average complete patient response rate (100% clearance of AKs of the face and scalp) was 70.8 ± 12.3% (mean ± 95% CI).
The results of this meta-analysis suggest that imiquimod has higher efficacy when compared with 5-fluorouracil for treatment of AKs of the face and scalp.
Discussion
Studies that determine the efficacy of a given treatment for a given disease can often report results that are not in agreement with other studies. A meta-analysis that combines the results of all studies that meet the inclusion criteria for a treatment strategy may be a helpful way of strengthening the evidence about that treatment.47–49
Although cryosurgery is the most common method of treating all types of actinic keratoses in all stages of development, it is generally limited to treating small areas and discrete lesions.5,6 Treating a large number of lesions, during any given clinical visit, may be painful or result in the development of adverse effects, e.g., blisters or pigmentary disturbance, thereby limiting the usefulness of this therapy. Thus, both imiquimod and 5-FU may have an advantage over cryotherapy especially when there is diffuse actinic damage rather than discrete AKs. Topical therapy is a useful alternative for treating this common skin condition and a number of preparations have been developed for treating AKs.50
Topical 5-FU has been a treatment for AK for almost 30 years, and it is therefore useful to compare the efficacy of 5-FU with the new nonsurgical therapy alternative, imiquimod. A number of studies have been conducted to determine the efficacy, safety, and optimal treatment regimens of 5-FU in the management of AK. 9,13,15,23,38,51–54 The mode of action of 5-FU is wellunderstood and relates to the depletion of thymidine resulting in a reduction of DNA synthesis in actively proliferating cells. This action leads to chemotoxic death of AK cells and can be associated with a local inflammatory reaction.16,55
In Canada imiquimod has been an approved treatment for external anogenital warts since 1999 and has recently received approval as treatment of AK. Imiquimod is an immune response modifier that stimulates local production of proinflammatory and immunostimulatory cytokines by innate immune cells, which in turn activates the adaptive immune response, eventually leading to apoptosis-mediated regression of the AK. This may be associated with a local inflammatory reaction which is generally less severe than that observed with 5-FU application.
This meta-analysis has several methodologic limitations. Imiquimod received U.S. and Canadian approval for treating AK in March and June 2004, respectively; therefore few studies have been published at the present time. In this study, we assessed the efficacy of topical fluorouracil and imiquimod creams for treatment of AKs on the face and scalp only. Some studies13,15,25–29 presented intent to treat results, whereby the entire initial randomized sample is included in the data presented, regardless of whether patients withdrew at any point during the trial. Others9,23,24 presented results as per protocol and excluded any data obtained from patients who withdrew from the study. The majority of studies included in the meta-analysis included any type of AK presented on the face and/or scalp, with the exception of Lebwohl et al.26 and Szeimies et al.24 who excluded hyperkeratotic and hypertrophic lesions. We may have missed some studies by not hand-searching all journals. However, a manual search was performed of all of the articles that we were able to retrieve. Studies with extractable data meeting the inclusion criteria for 0.5%, 1%, and 5% concentrations of 5-FU were pooled given the limited number of studies available for consideration for inclusion in the meta-analysis.
In summary, the results of this meta-analysis indicate that both 5-fluorouracil and imiquimod are effective methods for the treatment of actinic keratosis of the face and scalp and provide a useful alternative to cryotherapy. Our analysis suggests that imiquimod may have a higher efficacy than 5-FU, thus providing another option to dermatologists when treating AK lesions on the face and scalp.
References
Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990–1994. Arch Intern Med 1998; 158:726–730
Gupta AK. The management of actinic keratoses in the United States with topical fluorouracil: a pharmacoeconomic evaluation. Cutis 2002; 70:30–36
Moy RL. Clinical presentation of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol 2000; 42:8–10
Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol 2000; 42:4–7
Dinehart SM. The treatment of actinic keratoses. J Am Acad Dermatol 2000; 42:25–28
Drake LA, Ceilley RI, Cornelison RL, et al. Guidelines of care for actinic keratoses. Committee on Guidelines of Care. J Am Acad Dermatol 1995; 32:95–98
Feldman SR, Fleischer AB Jr, Williford PM, et al. Destructive procedures are the standard of care for treatment of actinic keratoses. J Am Acad Dermatol 1999; 40:43–47
Marks R. The role of treatment of actinic keratoses in the prevention of morbidity and mortality due to squamous cell carcinoma. Arch Dermatol 1991; 127:1031–1033
Dillaha CJ, Jansen GT, Honeycutt WM, et al. Further studies with topical 5-fluorouracil. Arch Dermatol 1965; 92:410–417
Gupta AK, Weiss JS, Jorizzo JL. 5-fluorouracil 0.5% cream for multiple actinic or solar keratoses of the face and anterior scalp. Skin Therapy Lett 2001; 6:1–4
Jansen GT. Topical therapy with 5-fluorouracil. J Surg Oncol 1971; 3:317–323
Jeffes EW III, Tang EH. Actinic keratosis. Current treatment options. Am J Clin Dermatol 2000; 1:167–179
Jorizzo J, Stewart D, Bucko A, et al. Randomized trial evaluating a new 0.5% fluorouracil formulation demonstrates efficacy after 1-, 2-, or 4-week treatment in patients with actinic keratosis. Cutis 2002; 70:335–339
Rossman RE. Topical fluorouracil therapy. South Med J 1969; 62:1240–1242
Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis 2002; 70:22–29
Eaglstein WH, Weinstein GD, Frost P. Fluorouracil: mechanism of action in human skin and actinic keratoses. I. Effect on DNA synthesis in vivo. Arch Dermatol 1970; 101:132–139
Dahl MV. Imiquimod: an immune response modifier. J Am Acad Dermatol 2000; 43:S1–S5
Dahl MV. Imiquimod: a cytokine inducer. J Am Acad Dermatol 2002; 47:S205–S208
Del Rosso JQ. An update on newer topical therapies for actinic keratoses: advances and applications. J Drugs Dermatol 2003; 2:35–39
Stanley MA. Mechanism of action of imiquimod. Papillomavirus Rep. 1999; 10:23–29
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188
Velanovich V. Meta-analysis for combining Bayesian probabilities. Med Hypotheses 1991; 35:192–195
Breza T, Taylor R, Eaglstein WH. Noninflammatory destruction of actinic keratoses by fluorouracil. Arch Dermatol 1976; 112:1256–1258
Szeimies RM, Gerritsen MJ, Gupta G, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol 2004; 51:547–555
Lawrence N, Cox SE, Cockerell CJ, et al. A comparison of the efficacy and safety of Jessner’s solution and 35% trichloroacetic acid vs 5% fluorouracil in the treatment of widespread facial actinic keratoses. Arch Dermatol 1995; 131:176–181
Lebwohl M, Dinehart S, Whiting D, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol 2004; 50:714–721
Shuttleworth D, Marks R. A comparison of the effects of intralesional interferon alpha-2b and topical 5% 5-fluorouracil cream in the treatment of solar keratoses and Bowen’s disease. J Dermatol Treat 1989; 1:65–68
Stockfleth E, Meyer T, Benninghoff B, et al. Successful treatment of actinic keratosis with imiquimod cream 5%: a report of six cases. Br J Dermatol 2001; 144:1050–1053
Stockfleth E, Meyer T, Benninghoff B, et al. A randomized, double-blind, vehicle-controlled study to assess 5% imiquimod cream for the treatment of multiple actinic keratoses. Arch Dermatol 2002; 138:1498–1502
Abadir DM. Combination of topical 5-fluorouracil with cryotherapy for treatment of actinic keratoses. J Dermatol Surg Oncol 1983; 9:403–404
Bercovitch L. Topical chemotherapy of actinic keratoses of the upper extremity with tretinoin and 5-fluorouracil: a double-blind controlled study. Br J Dermatol 1987; 116:549–552
Bianchi L, Campione E, Marulli GC, et al. Actinic keratosis treated with an immune response modifier: a ease report of six patients. Clin Exp Dermatol 2003; 28(Suppl 1):39–41
Chen K, Yap LM, Marks R, et al. Short-course therapy with imiquimod 5% cream for solar keratoses: a randomized controlled trial. Australas J Dermatol 2003; 44:250–255
Eklind J, Tartler U, Maschke J, et al. Imiquimod to treat different cancers of the epidermis. Dermatol Surg 2003; 29:890–896
Epstein E. Treatment of lip keratoses (actinic cheilitis) with topical fluorouracil. Arch Dermatol 1977; 113:906–908
Kurwa HA, Yong-Gee SA, Seed PT, et al. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol 1999; 41:414–418
Labandeira J, Pereiro M Jr, Valdes F, et al. Intermittent topical 5-fluorouracil is effective without significant irritation in the treatment of actinic keratoses but prolongs treatment duration. Dermatol Surg 2004; 30:517–520
Loven K, Stein L, Furst K, et al. Evaluation of the efficacy and tolerability of 0.5% fluorouracil cream and 5% fluorouracil cream applied to each side of the face in patients with actinic keratosis. Clin Ther 2002; 24:990–1000
Pearlman DL. Weekly pulse dosing: effective and comfortable topical 5-fluorouracil treatment of multiple facial actinic keratoses. J Am Acad Dermatol 1991; 25:665–667
Persaud A, Lebwohl M. Imiquimod cream in the treatment of actinic keratoses. J Am Acad Dermatol 2002; 47:S236–S239
Persaud AN, Shamuelova E, Sherer D, et al. Clinical effect of imiquimod 5% cream in the treatment of actinic keratosis. J Am Acad Dermatol 2002; 47:553–556
Robins P. Pulse therapy with 5-FU in eradicating actinic keratoses with less than recommended dosage. J Drugs Dermatol 2002; 1:25–30
Robinson TA, Kligman AM. Treatment of solar keratoses of the extremities with retinoic acid and 5-fluorouracil. Br J Dermatol 1975; 92:703–706
Salasche SJ, Levine N, Morrison L. Cycle therapy of actinic keratoses of the face and scalp with 5% topical imiquimod cream: An open-label trial. J Am Acad Dermatol 2002; 47:571–577
Smith S, Piacquadio D, Morhenn V, et al. Short incubation PDT versus 5-FU in treating actinic keratoses. J Drugs Dermatol 2003; 2:629–635
Warnock GR, Fuller RP Jr, Pelleu GB Jr. Evaluation of 5-fluorouracil in the treatment of actinic keratosis of the lip. Oral Surg Oral Med Oral Pathol 1981; 52:501–505
Cook DJ, Sackett DL, Spitzer WO. Methodologic guidelines for systematic reviews of randomized control trials in health care from the Potsdam Consultation on Meta-Analysis. J Clin Epidemiol 1995; 48:167–171
Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001; 323: 42–46
Mulrow C, Langhorne P, Grimshaw J. Integrating heterogeneous pieces of evidence in systematic reviews. Ann Intern Med 1997; 127:989–995
Silapunt S, Goldberg LH, Alam M. Topical and light-based treatments for actinic keratoses. Semin Cutan Med Surg 2003; 22:162–170
Levy S, Furst K, Chern W. A pharmacokinetic evaluation of 0.5% and 5% fluorouracil topical cream in patients with actinic keratosis. Clin Ther 2001; 23:908–920
Levy S, Furst K, Chern W. A comparison of the skin permeation of three topical 0.5% fluorouracil formulations with that of a 5% formulation. Clin Ther 2001; 23:901–907
Levy S, Furst K, Chern W. A novel 0.5% fluorouracil cream is minimally absorbed into the systemic circulation yet is as effective as 5% fluorouracil cream. Cutis 2002; 70:14–21
Simmonds WL. Topical management of actinic keratoses with 5-fluorouracil: results of a 6-year follow-up study. Cutis 1972: 10:737–742
Robins P, Gupta AK. The use of topical fluorouracil to treat actinic keratosis. Cutis x2002; 70:4–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by 3M Pharmaceuticals, London, Ontario, Canada.
Dr. Gupta has acted as an advisor and conducted clinical trials for 3M Pharmaceuticals, London, Ontario, Canada.
About this article
Cite this article
Gupta, A.K., Davey, V. & Mcphail, H. Evaluation of the Effectiveness of Imiquimod and 5-Fluorouracil for the Treatment of Actinic Keratosis: Critical Review and Meta-analysis of Efficacy Studies. J Cutan Med Surg 9, 209–214 (2005). https://doi.org/10.1007/s10227-005-0148-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10227-005-0148-6