Abstract
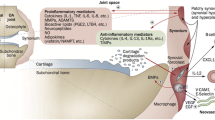
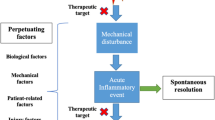
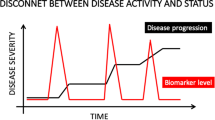
Significant advances have occurred in the symptomatic management of osteoarthritis over the past several decades. However, the development of so called disease-modifying osteoarthritis drugs is in a more formative stage. Although increased knowledge of osteoarthritis pathophysiologic pathways provides more rational opportunity for targeting specific elements of the degenerative process, limitations in our ability to measure disease progression/regression hamper assessment. Development of more sophisticated plain radiographic techniques and the use of additional technologies such as magnetic resonance imaging and gadolinium-enhanced magnetic resonance imaging of cartilage provide potential for more reproducible approaches. Noninvasive biomarkers that reflect structural change are the subject of intense investigation. Studies describing disease-modification effects provide optimism that disease prevention, retardation, and reversal are attainable.
Similar content being viewed by others
References and Recommended Reading
American College of Rheumatology SubCommittee on Osteoarthritis Guidelines: Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum 2000, 43:9:1905–1915. This set of guidelines sponsored by the American College of Rheumatology provides a comprehensive background of nonpharmacologic and pharmacologic approaches to management. These guidelines are meant to be recommendations and not rigid approaches to management.
Reginster JY, Bruyere O, Henrotin Y: New perspectives in the management of osteoarthritis. Structure modification: facts or fantasy? J Rheumatol 2003, 30(Suppl_67):14–20. This review is an excellent assessment of current concepts of disease modification in osteoarthritis and commentary on state of the science of disease modification.
Abadie E, Ethgen D, Avouac B, et al.: Recommendations for the use of new methods to assess the efficacy of disease-modifying drugs in the treatment of osteoarthritis. Osteoarthritis Cartilage 2004, 12:263–268. This article reviews currently available methods to assess the effects of disease-modification drugs in the treatment of osteoarthritis. JSN on plain radiographs is considered the technique of choice at this time; MRI may provide significant advances in terms of evaluating whole organ structures.
Dougados M, Gueguen A, Nguyen M, et al.: Radiological progression of hip osteoarthritis: definition, risk factors and correlations with clinical status. Ann Rheum Dis 1996, 55:356–362.
Brandt KD, Mazucca SA, Conrozier T, et al.: What is the best radiographic protocol for clinical trial of a structure modifying drug in patients with osteoarthritis. J Rheumatol 2002, 29:1308–1320.
Mazucca SA, Brandt KD, Buckwalter KA: Detection of radiographic joint space narrowing in subjects with knee osteoarthritis. Arthritis Rheum 2003, 48:385–390.
Conrozier T, Lequesne M, Favret H, et al.: Measurement of the radiological hip joint space width. An evaluation of various methods of measurement. Osteoarthritis Cartilage 2001, 9:281–286.
Vignon E, Conrozier T, Piperno M, et al.: Radiographic assessment of hip and knee osteoarthritis. Recommendations: recommended guidelines. Osteoarthritis Cartilage 1999, 7:434–436.
Altman RD, Bloch DA, Dougados M, et al.: Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis Cartilage 2004, 12:515–524.
Raynaud JP, Kauffman C, Beaudoins G, et al.: Reliability of quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis Cartilage 2003, 11:351–360.
Peterfy CG, Guermazi MD, Zaim MD, et al.: Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004, 12:177–190. This study emphasizes the opportunities to be obtained with using MRI which assesses not only cartilage, but other organ structures such as bone, muscle, synovium, and other soft tissues.
Otterness IG, Swindell AC, Zimmere RO, et al.: An analysis of 14 molecular markers for monitoring osteoarthritis: segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage 2000, 8:180–185. This is an excellent review of the current status of biomarkers in the study of osteoarthritis. The authors suggest that a "cluster" of biomarkers, taking into account aspects such as synthesis, degradation, and inflammation, may be required to assess joint responses.
Poole AR: Can serum biomarkers assays measure the progression of cartilage degeneration in osteoarthritis? Arthritis Rheum 2002, 46:2549–2552.
Mankin HJ, Dorfman H, Lippiello L, et al.: Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg 1971, 53A:523–537.
Poole AR, Howell DS: Etiopathogenesis of osteoarthritis. In Osteoarthritis: Diagnosis and Medical/Surgical Management. Edited by Moskowitz RW et al. Philadelphia: WB Saunders; 2001:29–47.
Brandt KD, Mazzuca SA, Conrozier T, et al.: Protocols for OA trials. Which is the best radiographic protocol for a clinical trial of a structure modifying drug in patients with knee osteoarthritis? J Rheumatol 2002, 29:1309–1320. This is an excellent review of caveats related to radiographic techniques in the study of structure-modifying drugs in osteoarthritis by a highly regarded leadership group in this field of study.
Mazzuca SA, Brandt KD, Dieppe PA, et al.: Effect of alignment of the medial tibial plateau and x-ray beam on apparent progression of osteoarthritis in the standing anterioposterior knee radiograph. Arthritis Rheum 2001, 44:1786–1794.
McAlindon TE, Watt I, McCrae F, et al.: Magnetic resonance imaging in osteoarthritis of the knee: correlation with radiographic and scientigraphic findings. Ann Rheum Dis 1991, 50:14–19.
Buckland-Wright J, Wolfe F, Ward R, et al.: Substantial superiority of semiflexed (MTP) views in knee osteoarthritis; a comparative radiographic study, without fluoroscopy, of standing extended, semiflexed (MTP), and schuss views. J Rheumatol 1999, 26:2664–2274.
Mazzuca SA, Brandt KD, Buckwalter KA, et al.: Field test of the reproducibility of the metatarsophalangeal view with repeated radiographic examinations of subjects with osteoarthritis of the knee. Arthritis Rheum 2002, 46:109–113.
Peterfy CG, Duryea J, Lynch JA, et al.: Nonfluoroscopic method for flexed radiography of the knee allows reproducible joint space measurement [abstract]. Arthritis Rheum 1998, 41(Suppl):S361.
Peterfy CG, Li J, Zaim S, et al.: Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint space width in the knee: testretest reproducibility. Skeletal Radiol 2003, 32:128–132.
Raynauld J-P, Martel-Pelletier J, Berthiaume M-J, et al.: Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum 2004, 50:476–487.
Tiderius CJ, Olsson LE, Leander P, et al.: Delayed gadoliniumenhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med 2003, 49:488–492.
Pessis E, Drape JL, Ravaud P, et al.: Assessment of progression in knee osteoarthritis: results of a 1-year study comparing arthroscopy and MRI. Osteoarthritis Cartilage 2003, 11:361–369.
Maillefert J-F, Dougados M: Is time to joint replacement a valid outcome measure in clinical trials of drugs for osteoarthritis? Rheum Dis Clin North Am 2003, 29:831–845.
Reginster JY, Deroisy R, Rovati LC, et al.: Long-term effect of glucosamine sulfate on osteoarthritis progression: a randomized placebo-controlled clinical trial. Lancet 2001, 27:251–265.
Pavelka K, Gatterova J, Olejarova M, et al.: Glucosamine sulphate delays progression of knee osteoarthritis: a 3-year, randomized placebo-controlled, double-blind study. Arch Intern Med 2002, 162:2113–2123.
Richy F, Bruyere O, Ethgen O, et al.: Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis. A comprehensive meta-analysis. Arch Intern Med 2003, 163:1514–1522.
McAlindon TE, LaValley MP, Gulin JP, et al.: Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 2000, 283:1469–1475. This is an excellent meta-analysis evaluating major well-performed studies with glucosamine and CS. The authors conclude that, despite caveats regarding trial methodology, these agents appear to have a positive effect size on osteoarthritis symptomatology.
Bruyere O, Pavelka K, Rovati LC, et al.: Glucosamine sulfate reduces osteoarthritis progression in postmenopausal women with knee osteoarthritis: evidence from two 3-year studies. Menopause 2004, 11:138–143.
Mazzuca SA, Brandt KD, Lane KA, et al.: Knee pain reduces joint space width in conventional standing anteroposterior radiographs of osteoarthritic knees. Arthritis Rheum 2002, 46:1223–1227.
Pavelka K, Bruyere O, Rovati LC, et al.: Relief in mild-tomoderate pain is not a confounder in joint space narrowing assessment of full extension knee radiographs in recent osteoarthritis structure-modifying drug trials. Osteoarthritis Cartilage 2003, 11:730–737.
Leeb BF, Schweitzer H, Montag K, et al.: A meta-analysis of chondroitin sulfate in the treatment of osteoarthritis. J Rheumatol 2002, 27:205–222.
Bucsi L, Poor G: Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow-acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis. Osteoarthritis Cartilage 1998, 6(Suppl):A31-A36.
Uebelhart D, Malaises M, Marcolongo R, et al.: Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthritis Cartilage 2004, 12:269–276.
Verbruggen G, Goemaere S, Veys EM: Systems to assess the progression of finger joint osteoarthritis and the effects of disease modifying osteoarthritis drugs. Clin Rheumatol 2002, 21:231–243.
Altman RD, Moskowitz R, and the Hyalgan® Study Group: Intraarticular sodium hyaluronate (hyalgan®) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol 1998, 25:2203–2212.
Sonoda M, Harwood FL, Wada Y, et al.: The effects of hyaluronan on the meniscus and on the articular cartilage after partial meniscectomy. Am J Sports Med 1997, 25:755–762.
Sonoda M, Harwood FL, Amiel ME, et al.: The effects of hyaluronan on tissue healing after meniscus injury and repair in a rabbit model. Am J Sports Med 2000, 28:90–97.
Toyoguchi T, Morihara T, Yamada K, et al.: Long-term effect of multiple courses of intraarticular injections of sodium hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage 2001, 9(Suppl B):S26.
Schiavinato A, Lini E, Guidolin D, et al.: Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. II. Morphological findings. Clin Orthop 1989, 241:286–299.
Listrat V, Ayral X, Patarnello F, et al.: Arthroscopic evaluation of potential structure modifying activity of hyaluronan (hyalgan®) in osteoarthritis of the knee. Osteoarthritis Cartilage 1997, 5:153–160.
Jubb RW, Beinat L, Piva S, et al.: A one-year randomized placebo (saline controlled trial of 500–730 KDA sodium hyaluronate (Hyalgan®) on the radiologic change in osteoarthritis of the knee. Inter J Clin Pract 2003, 57:464–474.
Martel-Pelletier J, Mineau F, Jolicoeur FC, et al.: In vitro effects of diacerhein and rhein on interleukin-1 and tumor necrosis factor-alpha systems in human osteoarthritis synovium and chondrocytes. J Rheumatol 1998, 25:753–762.
Moore AR, Greenslade KJ, Alam CAS, et al.: Effects of diacerhein on granuloma induced cartilage breakdown in the mouse. Osteoarthritis Cartilage 1998, 6:19–23.
Bendele AM, Bendele RA, Hulman JF, et al.: Beneficial effects of treatment with diacerhein in guinea pigs with osteoarthritis. Rev Prat 1996, 46:35–39.
Dougados M, Nguyen M, Berdah L, et al.: For the ECHODIAH investigator study group. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis. Arthritis Rheum 2001, 4:2539–2547.
Henrotin Y, Labasse A, Zheng SX, et al.: Effects of three avocado/ soybean unsponifiable mixtures on human articular chondrocyte metabolism. Clin Rheumatol 1998, 17:31–39.
Lequesne M, Maheu E, Cadet C, et al.: Structural effect of avocado/ soybean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Rheum 2002, 47:50–58.
Smith G Jr., Brandt KD, Mickler EA, et al.: Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritis cartilage. J Rheumatol 1998, 25:532–535.
Brandt KD, Mazzuca SA, Katz B, et al.: Doxycycline slows the rate of joint space narrowing in patients with knee osteoarthritis. Arthritis Rheum 2003, 48:SLB22.
Palmoski MJ, Brandt KD: Relationship between matrix proteoglycan content and the effects of salicylate and indomethacin on articular cartilage. Arthritis Rheum 1983, 26:528–531.
Palmoski MJ, Brandt KD: Effect of salicylate and indomethacin on glycosaminoglycan and prostaglandin E2 synthesis in intact canine knee cartilage ex vivo. Arthritis Rheum 1984, 27:398–403.
Rashad S, Hemingway A, Rainsford, et al.: Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet 1989, 2:519–522.
Huskisson EC, Berry H, Gishen P, et al.: Effects of anti-inflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal investigation of nonsteroidal anti-inflammatory drugs in knee osteoarthritis. J Rheumatol 1995, 22:1941–1946.
Jovanovic DV, Fernandes JC, Martel-Pelletier J, et al.: The in vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-300 reduces the progression of experimental osteoarthritis. Suppression of collagenase-1 and interleukin-1 beta synthesis. Arthritis Rheum 2001, 44:2320–2330.
Solchaga LA, Goldberg VM, Caplan AI: Experimental models of cartilage repair. Clin Orthop 2001, 391S:S161-S170.
Goldberg VM, Solchaga LA, Yoo JU, et al.: Chondroprogenitor cell repair of full thickness defects of articular cartilage. J Sports Traumatol Rel Res 1998, 20:81–99.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moskowitz, R.W., Hooper, M. State-of-the-art disease-modifying osteoarthritis drugs. Curr Rheumatol Rep 7, 15–21 (2005). https://doi.org/10.1007/s11926-005-0004-0
Issue Date:
DOI: https://doi.org/10.1007/s11926-005-0004-0