Article Text
Abstract
Objectives To compare prevalence and types of dispensing errors and pharmacists’ labelling enhancements, for prescriptions transmitted electronically versus paper prescriptions.
Design Naturalistic stepped wedge study.
Setting 15 English community pharmacies.
Intervention Electronic transmission of prescriptions between prescriber and pharmacy.
Main outcome measures Prevalence of labelling errors, content errors and labelling enhancements (beneficial additions to the instructions), as identified by researchers visiting each pharmacy.
Results Overall, we identified labelling errors in 5.4% of 16 357 dispensed items, and content errors in 1.4%; enhancements were made for 13.6%. Pharmacists also edited the label for a further 21.9% of electronically transmitted items. Electronically transmitted prescriptions had a higher prevalence of labelling errors (7.4% of 3733 items) than other prescriptions (4.8% of 12 624); OR 1.46 (95% CI 1.21 to 1.76). There was no difference for content errors or enhancements. The increase in labelling errors was mainly accounted for by errors (mainly at one pharmacy) involving omission of the indication, where specified by the prescriber, from the label. A sensitivity analysis in which these cases (n=158) were not considered errors revealed no remaining difference between prescription types.
Conclusions We identified a higher prevalence of labelling errors for items transmitted electronically, but this was predominantly accounted for by local practice in a single pharmacy, independent of prescription type. Community pharmacists made labelling enhancements to about one in seven dispensed items, whether electronically transmitted or not. Community pharmacists, prescribers, professional bodies and software providers should work together to agree how items should be dispensed and labelled to best reap the benefits of electronically transmitted prescriptions. Community pharmacists need to ensure their computer systems are promptly updated to help reduce errors.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Introduction
Studies from the UK1–4 and elsewhere5 ,6 have highlighted the prevalence of medication errors in primary care. Internationally, estimates of dispensing error rates in community pharmacies vary from 0.04%1 to 24%5 of dispensed items, the wide variation at least partly due to differences in methods and definitions. Dispensing errors may involve the wrong product being dispensed (‘content errors’) or the wrong information being printed on the dispensing label (‘labelling errors’). We previously used a research pharmacist to identify errors in nearly 3000 dispensed items in 11 community pharmacies in England and Wales.2 A content error was identified in 1.7% of dispensed items and a labelling error in 1.6%.
A commonly advocated approach to reducing dispensing errors is to make greater use of information technology such as computerised prescribing and the electronic transmission of prescriptions to community pharmacies for dispensing.7 ,8 While computerised prescribing has been commonplace in UK primary care for some time, until recently there has been no system for transferring prescriptions electronically to community pharmacies, and prescriptions were, instead, printed out and taken by hand. Within England, the introduction of the Electronic Prescription Service (EPS) began in 2005. This occurred in two phases: EPS Release 1 (EPSR1) and EPS Release 2 (EPSR2). EPSR1 was intended to test the infrastructure needed for EPSR2 in which electronic prescriptions are digitally signed by the prescriber and transmitted electronically to a nominated community pharmacy of the patient's choice. The first sites went live with EPSR2 in July 2009. Box 1 presents characteristics of each prescription type. As well as increased efficiency, the expected benefits of EPSR2 included gains in patient safety, as theoretically there is no need for pharmacy staff to re-enter a prescriber's instructions or interpret illegible prescriptions, thereby reducing the risk of errors involving transcription of information onto the dispensing label. However, it is not yet known whether these potential benefits have been realised. Internationally, most studies of electronic prescriptions in the community setting have focused on prescribing;9 ,10 there are few studies of the impact on dispensing errors. In a controlled before and after study, Moniz et al11 reported a reduction in the prevalence of dispensing errors from 3.1% to 1.8% of prescriptions when electronic transmission was added to a well-established electronic prescribing system in US outpatient clinics. However, the study was based on identifying discrepancies between electronic prescription records and electronic dispensing records; there was no check of dispensed items. Additionally, while there is a considerable literature on the interventions made by community pharmacists to resolve prescribing errors on paper-based and electronic prescriptions,12 ,13 little is known about other additional enhancements that community pharmacists make to clarify or enhance the prescriber's instructions on dispensing labels, and how these may change with the introduction of an electronic transmission system such as EPSR2.
Box 1 National Health Service (NHS) prescriptions dispensed in English community pharmacies
Non-EPS prescriptions are generally computer generated, printed onto an NHS prescription form, manually signed by the prescriber (who may be a medical or non-medical prescriber), and given to the patient to take to the pharmacy of their choice. Prescriptions are also occasionally handwritten directly onto the prescription form.
EPSR1 prescriptions are standard computer-generated paper prescriptions, manually signed by the prescriber as above, but with an additional barcode. These are given to the patient to take to the community pharmacy of their choice. If the barcode is scanned in an EPS-enabled pharmacy, an electronic copy of the prescription data can be downloaded to the pharmacy computer to populate the patient medication record and dispensing labels; alternatively the paper prescription can be dispensed as for a non-EPS prescription.
EPSR2 prescriptions are digitally signed by the prescriber and transmitted electronically to a nominated community pharmacy of the patient's choice. Patients are generally given a non-essential printed ‘token’ in lieu of a prescription, while the legal prescription is transmitted to the nominated pharmacy via a central server. Community pharmacies regularly download all prescriptions received. A barcode on the token can also be used to download the prescription at a different pharmacy if required.
In each case, one prescription form (or electronic equivalent) can generally include up to about four prescribed items; patients requiring more than this require more than one prescription form.
EPSR, Electronic Prescription Service Release.
Our objectives were to compare for EPSR2 versus non-EPSR2 prescriptions: (1) the prevalence and types of labelling and content errors in community pharmacies and (2) the prevalence of labelling enhancements made by pharmacy staff.
Methods
Setting
The study took place in a sample of English community pharmacies; the vast majority of prescriptions dispensed in such pharmacies were prescriptions from local general practitioners (GP) on standard National Health Service (NHS) prescription forms. We were interested in the three main types of NHS prescription (box 1).
Study design and sample size
The study was originally planned as a controlled before-and-after study. The prevalence of labelling errors was our primary outcome measure as these were anticipated to be reduced by EPSR2. The prevalence of content errors and labelling enhancements were secondary outcome measures. Our main comparison of interest was items prescribed using EPSR2 versus those prescribed on all other types of prescription (non-EPS and EPSR1). Exploratory work suggested that while EPSR1 prescriptions were computer generated, the electronic transmission functionality was rarely used and they were almost always taken by hand to the pharmacy; these were therefore grouped with non-EPS prescriptions for analysis. Our sample size was calculated based on the original controlled before-and-after design. We assumed a baseline labelling error rate of 1.6%,2 and anticipated a minimum 50% relative reduction in the mean labelling error rate in the EPS2 pharmacies. Assuming a reduction in the mean labelling error rate of 10% in the control group pharmacies, power of 80%, a 1 : 1 ratio of EPSR2:non-EPSR2 prescriptions, and a two-sided significance level of 5%, 26 pharmacies per group were required to detect a difference. After adjustment for clustering by primary care trust (PCT), assuming an intracluster correlation coefficient of 0.01, and that five pharmacies per PCT would be recruited, 28 pharmacies per group were required. We therefore aimed to recruit six PCTs in the EPSR2 group and six in a control group.
When it became apparent that the roll out of EPSR2 was beyond our control and that it would be difficult to assess the time point at which ‘after’ measurements should be assessed due to uncertainty about how quickly pharmacies would become proficient at using EPSR2, we revised our design to a non-randomised stepped wedge study. We estimated that within our existing resources we could collect data in 42 pharmacies, and that we would have 80% power to capture a change in the prevalence of labelling errors from 1.6%2 to 1.2% of dispensed items, (assuming a two-sided significance level of 5%, and that 200 dispensed items were examined at each of five data collection visits at each pharmacy site). We aimed to visit each pharmacy five times at approximately 3-month intervals, starting before the pharmacy had gone live with EPSR2.
Recruitment
Originally, it had been planned that EPSR2 would be launched in two phases of initial implementer pilot sites based in 17 English PCTs, prior to national roll out. We approached six PCTs due to be the first to go live and asked them to identify community pharmacies likely to go live with EPSR2 during the period 2009–2011. Pharmacies were approached and recruited on an on-going basis during this time. When these original implementation plans changed nationally, we continued collecting data at recruited sites even though some did not subsequently implement EPSR2 during our study period. We therefore started introducing new community pharmacy sites that had already started using EPRSR2 in order to obtain sufficient numbers of EPSR2 prescriptions; such pharmacies also dispensed non-EPSR2 prescriptions. We extended the data collection period to include more three-monthly data collection visits to compensate for having fewer pharmacies, and the uptake of EPSR2 being slower than expected.
Outcome measures
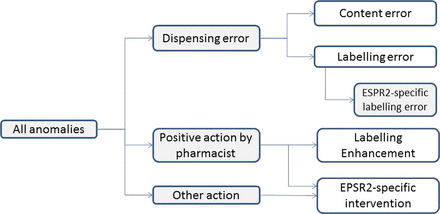
We initially identified ‘anomalies’ associated with dispensed items, where anomalies were any difference between the prescription plus any relevant statutory requirements, and the items dispensed. Anomalies included beneficial enhancements as well as potential dispensing errors, and each dispensed item could have more than one anomaly. Each anomaly was then classified into one or more of four mutually exclusive categories, indicated by the four white boxes in figure 1.
Classification of anomalies between prescription and dispensed items (EPSR2, Electronic Prescription Service Release 2).
We used a definition of a dispensing error developed with practising pharmacists for use in UK community pharmacies (box 2);2 this is accompanied by lists of examples of scenarios which should be included and excluded as errors. The Royal Pharmaceutical Society's Medicines, Ethics and Practice guide and the British National Formulary were used as references for statutory requirements and recommended additional labels, using the versions in use at the time of data collection. We also developed definitions for labelling enhancements and for EPSR2-specific labelling errors as we wished to identify these separately (box 2). Finally, pilot work revealed that for EPSR2 prescriptions, community pharmacy staff often made changes to the prescriber's directly transmitted directions to better communicate these to the patient. Where the original directions did not constitute a prescribing error, we termed these ‘EPSR2-specific interventions’ and reported these separately.
Box 2 Definitions
A dispensing error was defined as any unintended deviation from an interpretable written prescription or medication order. Any unintended deviation from professional or regulatory references, or guidelines affecting dispensing procedures, was also considered a dispensing error. Dispensing a medication without a patient information leaflet was not considered an error. Dispensing errors were subdivided into content errors and labelling errors, with EPSR2-specific labelling errors a further subcategory of labelling error. Content errors were those relating to the content (ie, the dispensed item itself or any additional consumables). Labelling errors were those concerning the dispensing label. An EPSR2-specific labelling error was any labelling error caused specifically by the different functionality of the EPSR2 system, when compared with a non-EPSR2 prescription.
A labelling enhancement was any deviation from an interpretable prescription, or from formal professional guidance and practice, which communicates beneficial information to the patient or caregiver (in addition to the statutory requirements), facilitates proper storage of the medicinal product provided, or enhances the dispensing process.
An EPSR2-specific intervention was any intentional modification of terminology between the prescriber's instructions on an EPSR2 prescription and that printed on the dispensed item's label, which was required specifically as a result of EPSR2 functionality, in order to reasonably fulfil the prescription as intended.
EPSR2, Electronic Prescription Service Release 2.
Data collection
Data collection methods were based on those used previously.2 A researcher (pharmacist or pharmacy technician) examined dispensed items in each pharmacy and recorded details of any anomalies. To minimise impact on pharmacy workflow, we focused on items previously dispensed and awaiting collection. However, if necessary to achieve the desired sample, and if practical, some items were also checked in real time, immediately after the pharmacist's final check and before they were given to the patient. Items dispensed against all types of NHS prescription (box 1) were included. Controlled drugs, items dispensed in multicompartment compliance aids and private prescriptions were excluded. Patient counselling was not observed. Data collection visits were agreed in advance with the relevant community pharmacist. At each data point in each pharmacy, we aimed to examine around 200 dispensed items over a 1 or 2-day period. Where more than 1 day's data collection was required to achieve this sample, these were scheduled up to 2 weeks apart to allow turnover of dispensed items awaiting collection.
On the first visit to each pharmacy, the researcher briefed the pharmacy staff regarding the aim of the study and the data collection methods. Pharmacy staff were informed that they would be alerted to errors if the researcher believed they would have a detrimental impact on the prescription's recipient, but that errors would not be reported to employers.
Dispensed items were compared against the prescription and a data collection form completed for each patient. For any dispensed item with one or more anomalies, details were recorded. If it was unclear whether an anomaly was intended or not, clarification was requested from the pharmacist. For example, 58 tablets dispensed against a prescription for 60 tablets could have been due to lack of stock with the balance to follow, rather than miscounting or misreading the prescription. If in doubt about any anomaly, the researcher recorded details for discussion among the wider team, as below.
Data were collected by four pharmacists and a senior pharmacy technician. The majority of data (79 of 95 data collection visits; 83%) were collected by the technician (MR) and/or one of the pharmacists (SS); these two researchers independently examined items dispensed for the same 40 patients at the beginning of the study to assess inter-rater reliability. The other three pharmacists were comprehensively briefed on methods and definitions, and shadowed one of the two primary researchers before commencing data collection themselves.
Classification
Initial classification of each anomaly was conducted by the researcher who collected the relevant data. Anomalies about which the researchers were uncertain, or which were potentially dispensing errors but not explicitly considered by Franklin and O'Grady,2 were discussed and classified by consensus during regular teleconferences attended by MR, SS, BDF, NB, MB and TA. A cumulative list of all discussions was produced, with classification decisions and principles recorded as a ‘case law’ document to ensure a consistent approach throughout the study. For example, it was agreed that if a prescriber specified an indication on the prescription (such as omeprazole ‘for oesophagitis’), then omission of this indication from the dispensing label was a labelling error.
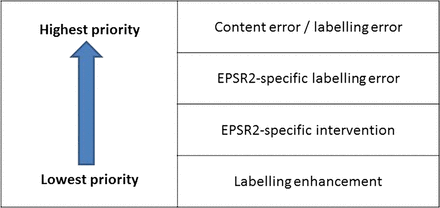
Once the main anomaly category (content error, labelling error, EPSR2-specific labelling error, labelling enhancement or EPSR2-specific intervention) was decided, each anomaly was further categorised into one of 16 mutually exclusive subcategories (see online supplementary appendix 1), based on those used previously.2 Database restrictions meant that only one anomaly of a given subcategory could be associated with each dispensed item. Very rarely, two or more anomalies with the same anomaly subcategory were identified in the same dispensed item; the hierarchy in figure 2 was then employed to determine which was recorded. For example, a dispensing label with a missing advisory label as well as an additional advantageous label would, in theory, require two classifications: ‘labelling error’ and ‘enhancement’. However, according to figure 2, a labelling error alone was recorded.
Anomaly classification hierarchy (EPSR2, Electronic Prescription Service Release 2).
Data entry and analysis
All data were entered into a MySQL database and checked by SS and MR to identify and resolve any inconsistencies in classification or data entry.
As well as descriptive analysis, two multivariable mixed effects logistic regression models were fitted for each outcome measure (labelling error, content error and enhancement) to explore differences in the prevalence of these outcome measures for EPSR2 in comparison to non-EPSR2 items after adjusting for pharmacy and time. The models were (1) EPSR2 versus non-EPSR2, adjusted for data collection visit (random effect) and pharmacy (fixed effect) and (2) EPSR2 versus non-EPSR2, adjusted for pharmacy characteristics (independent, small chain, etc) and location (inner city, suburbia, etc) as fixed effects, and data collection visit and pharmacy (both random effects). Both were two-level hierarchical models with the relevant outcome measure at level 1 and pharmacy at level 2, with non-EPSR2 items as the reference category. Effects of model variables were presented as ORs with associated 95% CIs; statistical significance was set at p<0.05 (two-sided). Inter-rater reliability for the two principal researchers’ assessment of 40 patients’ items was measured using Cohen's κ. Statistical analysis was conducted using STATA SE V.11.2 (STATA Corp).
Ethics
This study was considered a service evaluation by the Cambridgeshire I Research Ethics Committee, reference 08/H0304/58. Individual researchers obtained appropriate approvals from each local PCT; permission to attend each community pharmacy was given from the appropriate site lead. No patient-identifiable data were recorded.
Results
Overview
Nine pharmacies were approached and recruited initially, of which two were already using EPSR2 for a small proportion of prescriptions when data collection started. Two more went live with EPSR2 during our study period; the remaining five did not. A further six EPSR2-live pharmacies were subsequently approached and recruited. None of the pharmacies approached declined to take part. A total of 15 pharmacies were therefore studied in total, from across five English PCTs, using five different pharmacy computer systems. A further PCT was unable to assist with identifying pharmacies due to resource issues. All major general practice and pharmacy computer systems used in England were represented. Of the 15 pharmacies, 4 were from large national pharmacy chains, 8 were from smaller local chains and 3 were independent. Due to delays in national EPSR2 deployment, these numbers were much lower than our target sample of 42 pharmacies; the proportion of EPSR2 prescriptions in participating pharmacies was also lower than anticipated. We therefore conducted additional data collection visits in pharmacies wherever possible for the duration of the study.
Data collection took place between November 2009 and September 2012, with a gap from October 2011 to February 2012 inclusive, while a year-long project extension was negotiated to cover the delayed EPSR2 roll out. We conducted 95 data collection visits (mean 6.3 per pharmacy; range 3–11). The initial nine pharmacies were visited a mean of 7.8 times each; the six pharmacies recruited later were visited a mean of 4.2 times. We examined a total of 16 357 items prescribed on 8242 prescriptions for 6409 patients (not necessarily unique patients between data collection visits) across all data collection visits, with a mean of 549 prescription forms per pharmacy (range 203–961) and 172 items per visit.
Of the 16 357 dispensed items examined, about a quarter were prescribed on computer-generated non-EPS prescriptions (3654; 22%), 81 (0.5%) were handwritten, just over half were on EPSR1 prescriptions (8889 items; 54%), approximately a quarter (3699; 23%) were on EPSR2 prescriptions with a paper token, and 34 (0.2%) were on EPSR2 prescriptions sent without a paper token. Overall, 3733 items (23%) were therefore transmitted electronically. Online supplementary appendix 2 presents the cumulative numbers of EPSR2 and non-EPSR2 items examined during the study. The majority of items studied (91.7%) were already dispensed and awaiting collection: 8.3% were checked in real time.
Errors and enhancements
Overall, 885 (5.4%) of 16 357 items had a labelling error, 222 (1.4%) had a content error, and 2225 (13.6%) had an enhancement made by the community pharmacy staff. Of the 3733 EPSR2 items, an EPSR2-specific intervention was made for a further 817 (21.9%) to amend the directions as entered by the prescriber.
Types of labelling error are shown in table 1; these most commonly involved dose instructions and additional warning labels. There were 224 content errors in 222 items; these most commonly involved not providing a suitable measuring device for liquid medicines (124; 55%), or dispensing the wrong quantity of medication (75; 33%). The latter generally involved minor discrepancies between the label and the contents, such as a box of 30 tablets dispensed against a prescription for 28 tablets with a label stating 28 tablets. Enhancements made by community pharmacy staff largely concerned additional ancillary labels (over and above those recommended by the British National Formulary), but also included additional information included with the dose directions, or greater clarity in relation to the name of the product. EPSR2-specific interventions largely involved pharmacists translating shorthand directions (that the GP had typed and would appear verbatim on the dispensing label) into more patient-friendly versions, such as ‘1BD’ changed to ‘Take one tablet twice a day’; others involved changing the wording slightly to fit their preferred professional style. Examples of errors, enhancements and EPSR2-specific interventions are presented in box 3.
Number of errors of each subcategory of labelling error for non-EPSR2 and EPSR2 prescriptions
Box 3 Examples of errors, enhancements and interventions
Dispensing errors
Content errors
10 mg busipirone tablets prescribed; Buscopan 10 mg (hyoscine butylbromide) dispensed (labelled as for busipirone)
56 lansoprazole 15 mg capsules prescribed; one box of 28 dispensed in error
Labelling errors
Sulfasalzine 500 mg enteric coated tablets prescribed as ‘three to be taken twice daily’; labelled as ‘take three daily’
Aspirin dispensed without additional warning label 21 (‘take with or just after food, or a meal’) printed on the label
EPSR2-specific labelling errors
Co-dydramol 10/500 mg tablets prescribed with instructions ‘1–2 tabs 4–6 hourly prn/max 8 daily’ which was then printed verbatim on the dispensing label
Azithromycin 200 mg/5 mL suspension prescribed with instructions ‘take 3.25 mL (130 mg) on Monday, Wednesday and Friday. Please issue one bottle constituted and three bottles dry for mum to make up’ all of which was printed verbatim on the dispensing label
Labelling enhancements
Additional ancillary labels
Risedronate sodium 35 mg tablets—extra warnings added: ‘swallow whole’, ‘take with plenty of water’, ‘avoid milk, iron, zinc or indigestion remedies at the same time’ and ‘once a week dose’
Simvastatin tables—extra warning added: ‘avoid consumption of grapefruit’
Additional information in relation to dosing directions
Naproxen 500 mg tablets—prescription stated ‘As directed’. Labelled as ‘To be taken as directed by your doctor—usually one to be taken twice a day’
Citalopram 20 mg tablets—Prescription states ‘60 mg od’. Labelled as ‘Three to be taken (60 mg) daily’
Additional information surrounding product name
Prescribed warfarin 3 mg tablets—labelled as ‘warfarin 3 mg (blue) tablets’
EPSR2-specific interventions
Felodipine 10 mg MR (modified release) tablets prescribed ‘1 OM’ (once daily in the morning) and labelled as ‘one to be taken each morning’
EPSR2, Electronic Prescription Service Release 2.
The two principal data collectors examined the medication for 40 patients, comprising 85 dispensed items. κ was 0.85 for whether or not items had one or more errors, and 1.0 for whether or not there were any other anomalies.
The effects of EPSR2
Labelling errors were more prevalent for items prescribed on EPSR2 prescriptions, while the prevalence of content errors and enhancements were similar across all prescription types (table 2).
Prevalence of dispensed items with one or more labelling error(s), content error(s), enhancement(s) and EPSR2-specific intervention(s) for each type of prescription
The higher prevalence of labelling errors associated with EPSR2 prescriptions was due to errors of two subcategories: dose instructions and additional warning labels (table 1). The former mainly involved one pharmacy, where local GPs were in the habit of specifying the indication in the directions (eg, ‘One to be taken every morning for high blood pressure’) but this indication information was omitted from the dispensed item (eg, the label would simply become ‘One to be taken every morning’). The latter mostly involved out-of-date or incorrect additional warning labels, such as omeprazole capsules having an unnecessary warning to swallow whole.
In relation to the multivariable analysis, model 2 would not converge because the covariates relating to the pharmacy characteristics did not provide any additional information from that already given by adjusting for the pharmacy. Results from model 2 are therefore not presented. Table 3 presents the results obtained using model 1.
Results using multivariable model 1
This analysis indicated a statistically significant 46% increase (CI 21% to 76%) in labelling errors for EPSR2 items compared to non-EPSR2. We inspected the raw data to explore the cause of this difference and found that the largest absolute and relative percentage increase was attributable to one specific type of labelling error involving the omission of the indication, where specified by the prescriber, from the dispensing label. We identified 158 of these, 155 of which occurred at one pharmacy. While these occurred for EPSR2 and non-EPSR2 prescriptions, this pharmacy accounted for a relatively high proportion (15%) of EPSR2 items studied. We therefore conducted a sensitivity analysis, in which model 1 was rerun with all 158 cases of this nature coded as not being in error. This indicated no statistically significant effect of EPSR2 on labelling errors (table 3).
Discussion
Principal findings
Using an independent check of items dispensed in 15 English community pharmacies, we identified a labelling error in 5.4% of all items dispensed, and a content error in 1.4%. We also present for the first time the prevalence of labelling enhancements made by community pharmacy staff to better communicate information to the patient or caregiver or facilitate proper storage of the product, made for 13.6% of items. A further 21.9% of items dispensed from EPSR2 prescriptions received intervention on the part of community pharmacy staff to make the prescriber's electronically transmitted directions understandable, more patient-friendly and/or in line with that pharmacy's standard labelling format. When comparing items prescribed on EPSR2 versus non-EPSR2 prescriptions, we initially identified a higher prevalence of labelling errors for the EPSR2 items, but no difference in the prevalence of content errors or enhancements. The apparent increase in labelling errors was largely accounted for by errors at a single pharmacy in which the indication, where specified by the prescriber, was not included on the dispensing label. This occurred for EPSR2 and non-EPSR2 items. While this style of labelling is in line with good practice, these errors largely occurred at one pharmacy which accounted for a relatively high proportion of EPSR2 items, resulting in a disproportionately large influence on the findings relating to EPSR2 prescriptions. A sensitivity analysis in which these cases were not included as errors suggested that there was no remaining difference in labelling error rates.
Strengths and limitations
Strengths of our study include our large sample size, and our use of a gold standard data collection method involving examination of dispensed items by trained researchers with a pharmacy background. We used established methods and definitions, and a rigorous approach to the classification of errors and enhancements. Our study design is stronger than the before-and-after design used in previous studies,11 as each site acts as its own control. Limitations were that participating pharmacies were neither randomly selected nor allocated to order of adoption; they were innovators that mainly served one GP practice, and may not have been representative of other pharmacies. As the roll out was markedly slower than expected we recruited fewer sites than planned, conducting more visits per site to compensate. Our researchers could not be blinded to the type of prescription on which each item was prescribed, and 17% of data were collected by researchers for whom we did not test inter-rater reliability.
Comparison with previous literature
We report a similar prevalence of content errors (1.4% of dispensed items) to that of 1.7% published previously in a UK study using the same methods and definitions.2 However, the prevalence of labelling errors was higher in our study, affecting 5.4% of items in comparison with 1.6%.2 This was largely accounted for by errors involving the omission of medication indications, or use of additional warnings. Our error rates are also in line with international figures.1 ,5 Similar to previous qualitative studies,14 ,15 we also identified additional work required by pharmacy staff to improve quality of electronic prescriptions; in many cases community pharmacy staff had to translate directions to make these more patient-friendly.
Implications for practice
Our results suggest that one of the anticipated benefits of EPSR2, a reduction in labelling errors, is not currently being realised; this is predominantly because of prescribing practices and pharmacy computer systems. Prescribers’ dosage instructions vary in quality,4 and many prescriptions include dosage instructions that are not written directly for the patient's use. Instructions keyed in by the GP, such as ‘1OD’ (one to be taken daily), are automatically transferred by EPSR2 to the dispensing label; an amendment then has to be made manually by pharmacy staff, resulting in errors if this is not done or done incorrectly. The failure to write an appropriate instruction may reflect the GP's lack of knowledge of the consequences of their action, lack of knowledge of how to enter a quick prescribing code, poor usability of the software system, or a combination of all three. We recommend that community pharmacists, GPs, professional bodies and software suppliers work together to agree how items should be prescribed and labelled, how GP prescribing software can support this, and how this should best be communicated using EPSR2. Labelling errors caused by the pharmacy computer system usually involved the automatic inclusion of additional warning labels that were out of date, either because products had been reformulated, or because recommendations had been revised. Our data suggest that such problems were pharmacy-specific. Community pharmacists need to be aware of how and when to upgrade the databases used by their dispensing systems, and suppliers need to make this easy to do. The significant role of community pharmacy staff in improving labelling instructions, additional to their interventions involving contacting the prescriber, patient or other healthcare professional,16 is an important role which should be recognised.
Unanswered questions and future research
Future work should focus on how best to reap the potential benefits concerned with the electronic transmission of prescriptions. The severity of the errors should be considered in future work.
Conclusion
The introduction of EPSR2 did not seem to affect content errors nor, contrary to expectations, did it seem to reduce labelling errors. Additional work was required by pharmacy staff to improve the information on medication labels, some of which was a result of EPSR2. More than one in five items dispensed from EPSR2 prescriptions received intervention on the part of community pharmacy staff to make the electronically transmitted directions more understandable and/or in line with local practice. Community pharmacists and GPs need to work together to agree how items should be dispensed and labelled in order to best reap the benefits of EPSR2, and community pharmacists need to ensure that their computer systems are up to date. Community pharmacy staff play a key additional role in quality and safety, making enhancements to the labels of one in seven dispensed items, whether transmitted using EPSR2 or otherwise, to better communicate key information to the recipient.
Acknowledgments
We thank Melanie Lynn, Gill Gookey and Sarah Slight for assistance with data collection, Lorraine Buck for assistance with data entry, John Horton for designing the database, and participating community pharmacists, pharmacy and primary care trust staff for their assistance and support throughout the study.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online appendix 1
- Data supplement 2 - Online appendix 2
Footnotes
-
Contributors The study was conceived and designed by NB, AJA and BDF. SS and MR conducted data collection. Statistical analysis was conducted by SJA and RM; all authors played a substantial role in data analysis and interpretation. MR, SS, BDF, NB, MJB and AJA participated in the anomaly classification teleconferences. The paper was written by BDF with contributions from all authors. All authors approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have access to all of the data. NB is guarantor.
-
Funding This work forms part of independent research commissioned by the National Institute of Health Research (NIHR). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The CMSSQ is affiliated with the Centre for Patient Safety and Service Quality (CPSSQ) at Imperial College Healthcare NHS Trust which is funded by the NIHR as an NIHR Patient Safety Translational Research Centre. The funders played no role in study design, data collection, analysis, or interpretation of data, or in the writing of the article and the decision to submit it for publication.
-
Competing interests All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: other than the funding cited in the funding statement, the authors received no support from any organisation for the submitted work; BDF received travel expenses to speak at a meeting organised by the Guild of Healthcare Pharmacists which had sponsorship from suppliers of EP systems. At the time of the study, SS was also employed as a practice pharmacist within the former Nottinghamshire County Primary Care Trust. The authors declare no other financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
-
Ethics approval This study was considered a service evaluation by the Cambridgeshire I Research Ethics Committee, reference 08/H0304/58. Individual researchers obtained appropriate approvals from each local PCT; permission to attend each community pharmacy was given from the appropriate site lead. No patient-identifiable data were recorded.
-
Provenance and peer review Not commissioned; externally peer reviewed.
-
Transparency declaration The guarantor affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.