Article Text
Abstract
Aim To test the effectiveness of a tailored, pharmacist-led centralised advice service to improve adherence to patients on established medications.
Methods A parallel group randomised controlled trial was conducted. Patients prescribed at least one oral medication for type 2 diabetes and/or lipid regulation were eligible to participate. 677 patients of a mail-order pharmacy were recruited and randomised (340 intervention, 337 control). The intervention comprised two tailored telephone consultations with a pharmacist, 4–6 weeks apart, plus a written summary of the discussion and a medicines reminder chart. The primary outcome was self-reported adherence to medication at 6-month follow-up, collected via a postal questionnaire, analysed using generalised estimating equations. Secondary outcomes included prescription refill adherence, lipid and glycaemic control and patient satisfaction.
Results In intention-to-treat analysis 36/340 (10.6%) of the intervention group were non-adherent (<90% of medication taken in the past 7 days) at 6 months compared with 66/337 (19.6%) in the control group, yielding an unadjusted OR of 1.54 (95% CI 1.11 to 2.15, p=0.01). Analyses of dispensing data also showed that the odds of being classified as adherent (≥90%) were 60% greater for the intervention group compared with the control group (OR 1.60, 95% CI 1.14 to 2.24, p<0.01). In a subsample of patients who provided blood samples, glycaemic and lipid control did not differ significantly between groups (p=0.06 and p=0.24, respectively) but positive trends were observed. Ninety-two per cent of intervention group patients reported that they were satisfied with the service overall.
Conclusions A telephone intervention, led by a pharmacist and tailored to the individuals’ needs, can significantly improve medication adherence in patients with long-term conditions, using a mail-order pharmacy. Further work is needed to confirm a trend towards improved clinical outcome.
Trial registration number NCT01864239.
- Randomised controlled trial
- Patient-centred care
- Pharmacists
- Compliance
- Reminders
Statistics from Altmetric.com
Background
Self-administered medicines play an important role in managing many long-term conditions but research suggests that as many as 30–50% of patients do not take their medicines as prescribed.1–3 Non-adherence may be unintentional, for example, forgetting to take medication, or it may be an intentional decision not to take medicines or to alter the way in which it is taken. Non-adherence can have costly outcomes for the individual patient, health systems and society as a whole, and is of particular and growing importance against the current backdrop of ageing populations, with rising prevalence of long-term conditions and associated increases in the prescription of medicines. In 2003, WHO suggested that innovations to improve treatment adherence may have a greater impact on public health than any further developments in specific medical treatments.2 A recent estimate suggests that $269 billion could be saved globally each year through better adherence to medicines.4 However, although a vast literature has accumulated over several decades there remains a dearth of good quality conclusive evidence on how best to support medication adherence. A recent update of the Cochrane systematic review on adherence interventions found that just 5 out of 17 studies identified as having the lowest risk of bias successfully improved medication adherence and clinical outcomes; and no common characteristics associated with successful interventions were identified.5
Pharmacists increasingly provide a wide variety of interventions to support medication taking and there has been a growing body of research evaluating their role.6 A recent Cochrane systematic review on interventions to improve the safe and effective use of medicines identified interventions involving pharmacists in medicines management as having some evidence of positive impacts on adherence, medication problems and clinical outcomes but highlighted the need to strengthen the evidence base.7
In line with the approach advocated in the most recent update of the Cochrane systematic review of adherence interventions, the ‘Medicines Advice Service’ intervention in this study was adapted and refined from an intervention that had previously shown promise in a proof-of-concept randomised controlled trial (RCT), and which was subsequently developed from a centralised service into a nationally funded service in pharmacies in England, known as the New Medicine Service (NMS).8 ,9 A recently published large pragmatic RCT of the NMS reported that this pharmacist-led approach, focusing on individuals’ problems and concerns with newly prescribed medicines, significantly improved the odds of self-reported adherence 10 weeks after initiating the new medicine.10 The NMS intervention was determined to be cost-effective based on economic modelling of the likely health impact of improved adherence in the long term.10 While new medicines are an important target for intervention, they represent a tiny proportion of overall prescriptions within long-term conditions. A recent study found that just 0.25% of prescription items are eligible for NMS.11 Patients’ experiences of problems, concerns and information needs are not restricted to new medicines, and research has found that adherence tends to drop off over time.12 ,13 The aim of this study was to examine the effectiveness of this relatively brief intervention in patients already established on long-term medications for diabetes and/or lipid regulation, who use mail order pharmacy services.
Methods
Study design
The Medicines Advice Service Evaluation (MASE) was a single-site parallel group RCT of an intervention designed to improve medication adherence. Patients gave written informed consent prior to participation by returning a signed consent form by post to the lead author. The study took place between December 2012 and September 2014.
Setting and participants
Patients prescribed at least one oral medication for type two diabetes and/or lipid regulation were recruited through ‘Pharmacy2U’, a UK National Health Service (NHS)-contracted internet and mail-order pharmacy which has over 300 000 registered patients and works with over 2000 general practitioner (GP) practices throughout England. Exclusion criteria included being under the age of 18 years, living outside of England, difficulty reading or understanding English or understanding the details of the study, prescribed drugs for dementia or showing signs of cognitive impairment, substantial hearing or sight impairment, having medications ordered by a caregiver/family member, or receiving a first prescription of a medication included in the study. Eligible patients, identified through electronic searches of the pharmacy's database, were posted an invitation letter and information booklet by the pharmacy.
Randomisation and concealment
Participants were randomly allocated, in blocks of four, to either the intervention or control group, using a 1:1 ratio. Block randomisation was used to help ensure a balanced workload for the intervention pharmacist as participants were recruited over time. A random number sequence was computer generated by LW, and IL used the sequence to assign participants to a group in the order in which baseline questionnaires were returned. Neither author had any role in the delivery of the intervention. Due to the nature of the intervention it was not feasible to blind participants or pharmacy staff to the patients’ allocated group. To minimise potential reporting bias, the purpose of the study was presented as an opportunity to access advice from a pharmacist to address any issues associated with their medications, and did not refer to the concept of adherence.
Medicines advice service intervention
The Medicines Advice Service intervention is a pharmacist-led service, designed to be modifiable to support patients taking prescribed medication(s) for any long-term condition. In this study, the intervention was focused on lipid-lowering and antidiabetic medications. A report by the IMS Institute for Healthcare Informatics identified diabetes mellitus and hyperlipidaemia as having the highest avoidable costs within the US healthcare system.14 Both are significant risk factors for cardiovascular diseases, the most common cause of deaths from non-communicable disease worldwide, and are the leading contributors to the global disease burden in older people.15 ,16 Research suggests adherence to these drugs is poor and they were therefore considered important to target in this intervention study.17 ,18
The original intervention evaluated by Clifford et al8 and used in the NMS10 is described in the online appendix, along with the rationale for adaptations used in this study. The Medicines Advice Service intervention was comprised of three main components:
Two telephone consultations with a pharmacist, 4–6 weeks apart
A written summary of the discussion, posted to the patient after the first consultation
A medicines reminder chart, posted to the patient after the first consultation.
The telephone consultations followed a semistructured condition-specific interview guide. Consultations aimed to identify any particular problems or concerns that the patient may be having. Although consultations were designed to specifically target issues related to medication adherence and the interview guide was designed to focus on the medicines used in the selection criteria, the emphasis could be tailored to individuals’ specific needs, and a holistic approach was encouraged, taking into account any other prescribed or over-the-counter medications, or comorbid conditions. The pharmacist recorded any problems or concerns identified, and interventions provided, on a specially designed page on the pharmacy database. If the patient identified no particular issues, the pharmacist reinforced the importance of continuing to adhere to the prescribed medication regimen and offered healthy living advice (eg, dietary, weight loss, smoking cessation, etc). The follow-up phone call offered an opportunity to review any issues discussed in the initial consultation and to identify any new or outstanding problems to be addressed. At the end of the telephone consultation each participant was posted a letter summarising the key points discussed, and a personalised list of their current prescribed medications, known as a medicines reminder chart, indicating what each medicine is for, and how much of, and when, each should be taken.19
Standard care
Participants in the control group continued to receive their existing dispensing service, ordering prescribed medications online or by telephone, and having prescriptions delivered to their address. Patients ordering by telephone speak to customer care staff, although there were no restrictions on their ability to contact the pharmacist if they wished to. The pharmacy does not provide automatic refills but does operate a refill reminder service where patients are contacted by pharmacy staff when their repeat prescription is due for renewal and they can arrange for the prescription to be approved by the doctor and dispensed. Participants who had previously signed up to this service continued to do so, regardless of their group allocation. Participants in both groups continued to be treated according to the clinical discretion of their GP, hospital specialists or other healthcare professional. No formal arrangements were made to contact other healthcare providers about the trial.
Prior to the start of recruitment, pharmacy staff (including the pharmacist who delivered the intervention, and customer care staff who scheduled the intervention appointments) received training on the background and rationale for the study, the implementation of the study protocol, and the intervention and control group procedures. One fully qualified pharmacist, who had completed postgraduate training in independent and supplementary prescribing, delivered the intervention. All intervention calls were audio recorded and a small random selection (n=6) was reviewed and discussed within the research team early in the intervention phase. The intervention pharmacist subsequently received feedback from the research team and had an opportunity to discuss any challenges encountered. Regular contact was maintained with pharmacy staff throughout to provide ongoing support and to resolve any emerging issues.
Outcome measures and data collection
Self-complete postal questionnaires were used to collect data at baseline, and 4 weeks and 6 months after the follow-up telephone consultation (figure 1). Questionnaires were sent with a freepost return envelope, and a reminder letter was sent if the questionnaire was unreturned after 2–3 weeks. Biomedical data was collected at baseline and 6 month follow-up. Additional data extracted from pharmacy records included age, gender, number of items on repeat prescription, use of repeat prescription reminder service and medications dispensed. The primary outcome was self-reported adherence to lipid-lowering and/or oral diabetes medication(s), measured using questions from the Diagnostic Adherence to Medication Scale (DAMS).20 This short scale was developed following a systematic review of self-report adherence measures to address some of the gaps in existing measures.21 Content, face and preliminary construct validity has been established in a sample of 100 primary care patients taking a range of medicines for various conditions. Adherence ratings on the DAMS have been found to correlate with previously validated self-report measures.20 This instrument records the amount of each medication prescribed, and participants were asked to report how much of each they missed in the previous 7 days, to allow calculation of an adherence rate, either for one medicine individually or overall. The DAMS also includes a question to determine if any non-adherence reported is intentional, unintentional or both. Questions about taking too much medication were not included in this study. In the absence of a gold standard measure of adherence, a multimethod approach, including a self-report and another more objective measure, has been recommended.2 Therefore, we also included a measure of refill adherence. A medication possession ratio (MPR) was calculated from electronic pharmacy dispensing data. For each antidiabetes and lipid-lowering drug, the sum of the days’ supply was calculated from an index date to a final follow-up point (31 August 2014), and divided by the number of days within that range. For the control group, the index date was the first dispensing date after consent was received. For the intervention group, it was the date the first refill was dispensed after the first intervention phone call. Any day's supply dispensed for any period beyond the final follow-up was discounted when calculating the MPR. If a participant stopped or changed a medication it was censored from the last refill date. Maximum adherence was capped at 100%, even if a patient was dispensed more of a drug than they had been instructed to take.
Intervention and data collection timeline.
Participants were also asked to provide an optional blood sample at baseline and 6 month follow-up, using a self-administered finger prick test. Samples were posted to a central accredited laboratory where they were analysed. HbA1c was measured by a high-performance liquid chromatography using a cation exchange column. Total cholesterol was measured using enzymatic colorimetric methods.
On a 4 week follow-up self-report questionnaire, participants in the intervention group were asked to rate their agreement with the statement “Overall, I was satisfied with the Medicines Advice Service” on a 7-point Likert scale (1=very strongly agree, 7=very strongly disagree).
Data on the length of consultation calls was accessed from audio recordings. A random sample of 10% of pharmacy consultation records (n=31) was also accessed and analysed descriptively to explore the content of the intervention and possible mechanisms of action.
Sample size and statistical analysis
Based on a similar pharmacy intervention study, a sample size calculation was carried out estimating a reduction in the proportion of patients who self-report non-adherence from 15% to 7.5%.8 With 80% power and a two-sided significance level of 0.05, the required sample size was determined to be 278 participants in each group. Assuming a 10% dropout, we aimed to recruit approximately 612 individuals.
Adherence data is typically highly positively skewed and therefore the self-report and refill adherence data were dichotomised using a threshold of 90%. This cut point was chosen as it is inclusive in detecting non-adherence and has also been found to be significantly associated with health outcomes.23 ,24
The primary analysis was performed according to the intention-to-treat (ITT) principle to minimise potential bias from missing responses. Multiple imputations, using the fully conditional specification method, was used and 20 complete imputed data sets were created. The imputation model included the primary outcome (self-reported non-adherence at 4 weeks and 6 months post intervention), which was missing for 15.5% and 19.2% of the sample, respectively. Other variables included had no more than 4% missing data. The online appendix provides further details on the handling of missing values. Generalised estimating equation (GEE) analyses were performed. There were no differences in adjusted GEE analyses (adjusted for age, gender, therapeutic group, number of prescription items, reminder service, baseline adherence), therefore only unadjusted analyses are presented here.
In secondary analyses, χ2 tests were also used to compare the proportion of adherent and non-adherent individuals between the intervention and control groups at single time points (4 weeks and 6 months). Logistic regression was used to determine the odds of adherence in each group compared with the other. Participants were also categorised according to whether or not they met guideline recommended targets for cholesterol and HbA1c levels, and χ2 tests were used to detect any significant differences between groups. All analyses were carried out using SPSS V.21,25 and all statistical tests were two-sided adopting a 0.05 significance level.
Results
Participants
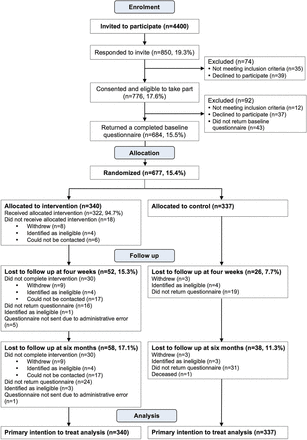
A total of 785 patients (17.8%) agreed to participate in the study by returning a signed consent form. At baseline, 684 patients were eligible and returned a completed questionnaire. Seven were excluded or withdrew prior to randomisation, leaving 340 in the intervention group and 337 in the control group. The flow of participants through the study is outlined in figure 2. Table 1 presents the baseline characteristics of the 677 participants who were randomised. The intervention and control groups were similar in terms of all sociodemographic, clinical and medication-related variables measured at baseline.
Baseline characteristics of participants
Flow of participants through the study.
Delivery of the intervention
Ninety-two per cent of the intervention group (n=310) completed the intervention. Eighteen people (5%) did not receive either telephone consultation, and a further 12 received the first telephone consultation but did not complete the follow-up consultation. The most common reasons for non-completion were not answering multiple calls and/or responding to messages to arrange an appointment, and not answering at the time of the appointment. Several attempts were made to contact participants and to reschedule missed appointments. Initial consultation calls lasted a median of 16 min 40 s (IQR 13–22 min). The median length of the follow-up consultations was 5 min 36 s (IQR 3–8 min). The median length of time between consultations was 35 days (IQR 28–41 days).
Content of the intervention
Of 31 randomly selected cases for which pharmacy records were accessed, 26 were consultations for cholesterol medicines only, 1 was for a consultation related to diabetes medication only, and 4 consultations related to cholesterol and diabetes medicines. In two-thirds of the first consultation calls sampled, the pharmacist recorded no problems, concerns or information needs. For the remaining 10 participants, 23 issues were recorded. The most common problem recorded was a potential side effect (n=7, 22.6%), followed by a need for more information about medicines (n=4, 13.3%). Table 2 illustrates some examples of the types of issues and outcomes recorded by the pharmacist during consultations.
Examples of issues and outcomes extracted from a random sample of pharmacist records of telephone consultations
Effect of the intervention on self-reported non-adherence
Four weeks and 6 months after intervention the intervention group had significantly better self-reported adherence (defined as ≥90% of medication taken in the previous 7 days) compared with the control group, across all analyses (table 3). According to the primary ITT GEE analysis, the intervention group had 54% increased odds of being adherent compared with the control group (OR 1.54, 95% CI 1.11 to 2.15, p=0.010). Complete case and available case analyses produced less conservative estimates. The results of adjusted and unadjusted logistic regression analyses at 4 week follow-up and 6-month follow-up are presented in table 3.
Unadjusted and adjusted effect of the intervention on self-reported non-adherence measured using the Diagnostic Adherence to Medication Scale at 4 week follow-up and 6 month follow-up
Effect of the intervention on pharmacy refill adherence
Analyses of dispensing data drawn from pharmacy records showed that over a longer follow-up period, averaging 434 days, 88/294 (29.9%) of the intervention group were categorised as non-adherent (<90% of medication available) compared with 127/313 (40.6%) of the control group, yielding an OR of 1.60 in favour of the intervention group (95% CI 1.14 to 2.24, p=0.006).
Effect of the intervention on glycaemic and lipid control
Valid results were obtained from 169 cholesterol tests and 41 HbA1c tests at follow-up. Six months after intervention, the intervention group had twice the proportion of participants achieving HbA1c less than 7% (66.7%, n=16) compared with the control group (31.3%, n=5), with this difference approaching statistical significance (p=0.061). The proportion of patients meeting guideline targets for total cholesterol levels (<5 mmol/L) was also higher in the intervention group (65.3%, n=64) compared with the control group (55.1%, n=38) at follow-up, although the differences were not statistically significant (p=0.24).
Satisfaction with the Medicines Advice Service
On the whole satisfaction with the Medicines Advice Service was high, with 91.8% (n=245) agreeing that they were satisfied overall. Just four participants (1.5%) expressed any dissatisfaction with the service. Two did not give a reason for dissatisfaction. One participant stated that the service was not really useful as he had no concerns about his medicines and one other participant described a problem with the pharmacy's services in general rather than with the Medicines Advice Service in particular. Regression analyses identified no significant predictors of overall satisfaction. The majority of participants (81.6%, n=217) agreed that they would recommend the service to someone else in their position.
Discussion
The MASE RCT has shown that mail-order pharmacy patients who participated in a tailored intervention delivered by a pharmacist had significantly increased odds of adherence, measured using self-report and pharmacy refill data, compared with a usual care control group. The findings were robust, producing consistent effects in ITT and complete case analyses.
The magnitude of the effect on self-reported adherence in our study is consistent with similar community pharmacy-based interventions in a variety of long-term conditions in the UK.8 ,10 ,26 The study also provides insight into the effect of pharmacist intervention on medication refill adherence. Studies using dispensing data are relatively common in the USA but this is one of the first UK studies to examine adherence based on pharmacy prescription refill rates. This approach allows for objective assessment of the effect of the intervention over a considerably longer period of time, compared with previous studies.8 ,10 The findings indicate that this relatively brief intervention can have a sustained impact on adherence. However, the proportion of patients classified as non-adherent according to dispensing data was higher than indicated in self-report data. It may be that the discrepancy reflects real differences in adherence in the time periods each measure covers (7 days compared with mean 434 days). Alternatively, social desirability bias in self-report may have resulted in an overestimation of the true level of adherence, and/or refill adherence may be underestimated for the one-fifth of patients in this study who reported using another pharmacy at least once to collect their diabetes or cholesterol drugs.
Glycaemic and lipid control were also assessed as objective markers of clinical impact, and non-significant improvements in the proportion of patients meeting guideline targets were observed in the intervention group compared with the control group. However, the study used a novel method of collecting blood samples at a distance and many patients reported difficulty drawing a sufficient sample of blood using the finger prick testing kit supplied. The number of samples available for analysis at follow-up was therefore small and further appropriately powered research is required to determine whether these findings are reliable and clinically meaningful. Most, though not all, reviews have identified improvements in cholesterol and glycaemic levels associated with pharmacist intervention.27–30
High rates of intervention completion indicated that the service was also acceptable to patients. Data collected on patients’ satisfaction and willingness to recommend the service support this view. These findings are largely consistent with other studies looking at patients’ perspectives on pharmacist intervention.31 ,32
Strengths and limitations
This large randomised controlled trial, incorporating data from multiple sources (patient self report, pharmacy records, blood results) and exploring outcomes over considerably longer follow-up periods, strengthens and extends previous findings on the efficacy of tailored pharmacist intervention in improving adherence to long-term medications. However, there are a number of limitations. First, although the required sample size was exceeded in this trial, a large number of eligible patients were invited to take part, and the positive response rate was just over 17%. In order to explore the possibility of recruitment bias, the MASE study sample was compared with anonymised data supplied by the pharmacy on four variables: age, gender, number of items on repeat prescription and uptake of the pharmacy's repeat prescription reminder service. No major differences were found in the characteristics of those who agreed to take part compared with the overall eligible population on these variables.
Although the intervention was developed using the available evidence and theory, and key elements had been tested in previous research, these were applied in a novel setting, and additional new components and methods of data collection may well have benefitted from a feasibility and piloting stage, as recommended in the Medical Research Council guidelines on developing and evaluating complex interventions.33 This may have helped to identify methods to improve the initial response rate and the return of viable blood samples.
Participants were recruited from a single mail-order pharmacy and it is possible the results may not be generalisable to other settings. Recent database studies from the USA have suggested that use of mail-order pharmacy is associated with better refill adherence and health outcomes when compared with regular brick and mortar pharmacies.34 ,35 Patients in the UK are free to obtain their NHS prescriptions from any pharmacy. No published research has explored the characteristics of patients who opt to use mail-order pharmacy. A comparison of our baseline demographic and clinical data with other UK studies suggests that patients who use mail-order pharmacy in the UK may not differ significantly from those using regular community pharmacy services.8 ,10 ,36–38
A third limitation linked to generalisability, was the delivery of the intervention by just one pharmacist, who holds a postgraduate qualification in independent and supplementary prescribing, and may have been particularly motivated and enabled to consistently and rigorously deliver the intervention. Further testing of the intervention with a wider group of pharmacists would be beneficial. However, the widespread implementation of similar services such as the NMS and Medicines Use Reviews in community pharmacies, suggests that this type of intervention is transferable to different settings and pharmacists.
Implications for practice and future research
To the best of our knowledge this is the first UK study to explore the impact of pharmacist intervention in a cohort of patients using mail-order pharmacy. Internet and mail-order pharmacy is a relatively new phenomenon in the UK and represents a small but growing proportion of pharmacies.39 The results of this trial demonstrate that it is feasible and effective to provide adherence support to mail-order pharmacy users, using a combination of telephone consultations and written information. Further research is needed to establish cost-effectiveness in the mail-order setting. Previous work with similar interventions suggests that this is a cost-effective approach, with improvements in adherence translating into modest healthcare gains and cost savings in the long term.9 ,10 Although the mail-order context has some unique characteristics, parallels between this intervention and existing services such as NMS suggest that this approach could be feasibly implemented within community pharmacy settings, or that these services could be extended and adapted to better facilitate mail-order pharmacies. Indeed, evidence from the evaluation of the NMS, along with findings from the PharmOutcomes database indicates that even in a community pharmacy setting telephone consultations are considered convenient and appropriate, with the majority of NMS interventions delivered by telephone.10 ,40 ,41
Conclusion
The results presented here have shown that tailored intervention, delivered by a pharmacist, comprising spoken information and advice by phone and written information by post, was acceptable to patients, and was effective in significantly improving medication adherence in a mail-order pharmacy cohort. These results are robust, using two methods of measuring adherence, and the effect is still present more than 6 months after the intervention. The findings provide further support for the enhanced role of pharmacists in supporting and advising patients with their medicines to improve outcomes.
Acknowledgments
The authors would like to thank all the staff at Pharmacy2U who helped to facilitate this research. In particular we would like to thank Dr Julian Harrison, for his collaboration and contribution to the conception and design of the study, and the development of the intervention, and Mr Phil Day, who helped develop the intervention content and delivered the intervention. We are also grateful to Gill Dorer, a patient representative, who provided feedback on the study protocol and materials. Finally, we would like to gratefully acknowledge all the individuals who gave their time to participate in this research.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Contributors NB, DKR and IL designed the study. IL carried out the data analyses, with input from LW, and drafted the manuscript. All authors contributed to editing and revising of the manuscript, and approved the final manuscript.
Funding This work was jointly funded by the UCL School of Pharmacy and Pharmacy2U as part of an initiative from the UCL School of Pharmacy which half funds PhD students with industry providing the other half. Pharmacy2U had no role in the analysis or interpretation of the data, or in the preparation or review of the manuscript.
Competing interests DKR is co-founder and academic advisor to Luto Research which develops, refines and tests health information materials. All other authors declare that they have no competing interests.
Ethics approval The London Brent Research Ethics Committee (12/LO/1657) and the NHS Leeds R&D Office (L001_22_11_12_0000).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data collection questionnaires are available on request from the corresponding author.