Abstract
Purpose To assess the quality of processed ophthalmic instruments and look for the presence of foreign material on the surface of these instruments.
Methods Data were prospectively collected on the presence of debris on processed instruments in the trays used for phacoemulsification surgery. All instruments were examined under an operating microscope before use and details of the types of debris on the various instruments were noted. If debris was found, a new tray was opened to obtain a clean instrument.
Results Forty-seven trays were opened for use during the study period. Deposits on instruments were found in 29 (62%) trays. These were mainly present on the intraocular lens introducers. Loose fibres were found on instruments from eight (17%) trays. Debris was found in the aspiration channels of three (6%) hand pieces.
Conclusions A significant number of processed ophthalmic instruments had debris on their surfaces. To reduce the risk of intraocular inflammation and of transmission of prion diseases the instruments should go through a thorough decontamination process before sterilization. Routine mechanical cleaning at the end of surgery and ultrasonic cleaning before sterilization should reduce the occurrence of debris on the instruments. Instruments should also be inspected under the operating microscope before use.
Similar content being viewed by others
Introduction
Introduction of foreign material into the eye can trigger an inflammatory reaction. This can cause endophthalmitis in the postoperative period. Debris on instruments also puts patients at risk of cross infection. We present our data on foreign materials that were present on processed ophthalmic instruments forming part of the trays used for phacoemulsification surgery.
Methods
Data were prospectively collected on debris present on processed ophthalmic instruments. These instruments were presented to the surgeon for use during phacoemulsification surgery. The surgeon inspected every instrument under the operating microscope before using it. A record of the types of debris present on the various instruments was kept. If any instrument was unusable, a new tray was opened for use.
Results
Forty-seven trays were opened for phacoemulsification surgery during the study period. Deposits on the instruments were found in 29 (62%) trays. These were mainly on the intraocular lens (IOL) introducers. These deposits were present on the inner surfaces of the tips, joints and the notches at the tips of the forceps. Similar deposits were found on the tips of the IOL folders. In addition, loose fibres were found adherent to the tips of the IOL folders, IOL introducers and on the tips of the irrigation and aspiration (I/A) hand pieces. These were found on instruments from eight (17%) trays. Debris was found extruding from the aspiration ports of three (6%) I/A hand pieces. The presence of debris in the I/A hand pieces was identified by flushing the aspiration channels by engaging the reflux mechanism on the foot pedal of the phacoemulsification system.
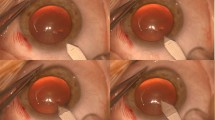
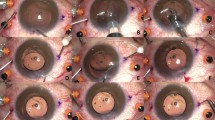
The various types of debris found on the instruments are shown in Figures 1–7. These debris were found on processed instruments that were inspected before use. They had been subjected to the usual cleaning and sterilisation process. The amount of deposit on a single instrument can be extensive. Figure 3 shows the deposit removed from the inner surfaces of the tips of a single IOL introducer. In one instance a fibre was found adherent to a silicone intraocular lens when inspected before insertion (Figure 8).
Discussion
Intraocular inflammation may be caused by retention of foreign material like cotton fibres after intraocular surgery or from introduction of material like starch into the eye at the time of surgery.1,2 Debris from instruments can enter the eye if the surgeon is unaware of its presence.
Intraocular inflammation and bullous keratopathy were reported by Kim3 following cataract surgery. This was thought to have been caused by the denatured sodium hyaluronate in the reusable cannulas. The denaturation was caused by the disinfection and sterilization process. The hypothesis was proved by inducing intraocular inflammation in rabbit eyes by injecting denatured sodium hyaluronate. Sutphin and Papadimus4 reported postoperative keratitis attributing it to sterilized viscoelastic material trapped in reusable cannulas. Kaufman et al5 found debris on sterile blades used to make laser in situ keratomileusis (LASIK) flaps using a scanning electron microscopy technique. In an experimental study they found debris and inflammatory cells in the LASIK interfaces of corneas of the rabbits when these blades were used.
Deposits like those in Figures 1–3 were found mainly on the IOL introducers. The exact nature of the material that is deposited on the surfaces of these instruments remains unclear. It may be possible that it is the viscoelastic substance to which the introducer is exposed, when in the anterior chamber during the insertion of the IOL into the capsular bag. Drying of the viscoelastic material may have made it difficult to remove during the routine cleaning and sterilization process. Des Coteaux et al6 found residual organic debris on processed surgical instruments like endoscopes and other conventional instruments used in general surgical procedures. Although on visual inspection 90.6% of instruments appeared clean, inspection with a micro-photographic system showed presence of debris on 84.3% of instruments. Mere visual inspection of instruments is not adequate to recognize these deposits. Microscopic inspection of instruments after decontamination should identify inadequately processed instruments.
Loose debris like the fibres on the tips of instruments (Figures 4–7) could have been attracted to them by static forces. Similarly, the fibre on the optic of the IOL (Figure 8) could have been attached to it from the surface it was laid on at the time of folding. Foldable lenses should be laid on fibre-free surfaces when folding. The sheets used to cover the surface of the instrument trolley should be made of fibre-free material and the instruments should only rest on surfaces that do not have such fibres.
In this study the deposits were predominantly found on the surfaces of the forceps that grasp the foldable IOL. It is important to inspect the instruments under the operating microscope to identify the presence of debris. Once identified, the loose debris like the fibres should be removed before using the instrument. Clean instruments from another tray should be used if deposits were found. The silicone material may allow adhesion of bacteria7 and other biomaterials. The foreign material from an instrument can get deposited on the surface of the IOL and gain entry into the eye. The sterile material may retain its antigenicity and initiate an inflammatory reaction. Identifying and avoiding entry of foreign materials into the eyes is important to prevent intraocular inflammation.
The presence of organic debris on instruments interferes with the sterilization process as they insulate the organisms from coming in contact with the killing agent.8 This may put the patient at the risk of developing intraocular infection. There are also concerns that prions may be transmitted iatrogenically via contaminated surgical instruments as the prions resist routine sterilizaton.9,10 There has been a lot of interest recently in the transmissible spongiform encephalopathies or prion diseases which are a group of neurodegenerative diseases. The variant Creutzfeldt–Jakob disease (vCJD) is a human prion disease that is agreed to be a human manifestation of Bovine Spongiform Encephalopathy (BSE) in cattle.11 Evidence from case reports suggests that prion diseases can be transmitted via surgical instruments.12 The infective agent of prion diseases is resistant to conventional sterilization techniques. The only generic process that is known to be effective in reducing the risk of transmission of prions is the removal of traces of organic material from the instruments during the decontamination process.13 Ophthalmic practice has attracted special interest in the transmission of vCJD.14 Thorough decontamination of ophthalmic surgical instruments is vital to reduce the risk of transmission of prion diseases.
Beran15 carried out a study in which the diamond blades used for refractive surgery were subjected to a graded cleaning process. He found that after irrigation with water, only 2/50 instruments were clean, after ultrasonic and detergent cleaning, 41/48 were clean and after mechanical styrofoam block cleaning, the remaining 7/7 were found to be clean. Ultrasonic cleaning of ophthalmic instruments should be a routine to facilitate adequate removal of deposits from the surfaces of instruments. Pressure syringing of the aspiration channels of the I/A hand pieces should be carried out at the end of the operation to remove the debris before they dry up. Prevention of the deposits on the surface of instruments is possibly the best way of eliminating denatured residue as a source of sterile endophthalmitis. Viscoelastic substance from the tips of the introducers should be removed by soaking the instrument in water and using a soft brush to clean the surfaces and the crevices. This should be carried out in the operating theatre before the instruments are sent for sterilization. Dried debris is more difficult to remove.
Conclusion
Processed ophthalmic instruments have debris on them that may gain entry into the eyes at the time of intraocular surgery. This may be responsible for intraocular inflammation and infection. These instruments also pose a risk of transmission of infective agents of prion diseases. Every possible measure should be undertaken to ensure the cleanliness of the instruments before using. The measures that we suggested may help in preventing the deposits from occurring. The answer may lie in processing ophthalmic instruments through routine ultrasonic cleaning and using a chemical process to remove the debris before sterilisation. Simple measures like mechanical cleaning of the instruments, especially those that are prone to develop deposits, in the operating theatre should help to avoid this problem. The surgeon must still be vigilant and inspect instruments under the operating microscope before use.
References
Kresloff MS, Castellarin AA, Zarbin MA . Endophthalmitis. Surv Ophthalmol 1998; 43: 193–224
Cox MJ, Woods JA, Newman S, Edlich RF . Toxic effects of surgical glove powders on the eye. J Long Term Eff Med Implants 1996; 6: 219–226
Kim JH . Intraocular inflammation of denatured viscoelastic substance in cases of cataract extraction and lens implantation. J Cataract Refract Surg 1987; 13: 537–542
Sutphin JE, Papadimus TJ . Post-cataract extraction corneal edema: epidemiological intervention and control. Invest Ophthalmol Vis Sci 1989; 30 (Suppl): 165
Kaufman SC, Maitchouk DY, Chiou AG, Beuerman RW . Interface inflammation after laser in situ keratomileusis. Sands of the Sahara syndrome. J Cataract Refract Surg 1998; 24: 1589–1593
Descoteaux JG, Poulin EC, Julien M, Guidoin R . Residual organic debris on processed surgical instruments. AORN J 1995; 62: 23–30
Gabriel MM, Ahearn DG, Chan KY, Patel AS . In vitro adherence of Pseudomonas aeruginosa to four intraocular lenses. J Cataract Refract Surg 1998; 24: 124–129
Miller CH . Cleaning, sterilization and disinfection: basics of microbial killing for infection control. J Am Dent Assoc 1993; 124: 48–56
Taylor DM . Inactivation of SE agents. Br Med Bull 1993; 49: 810–821
Frosh A, Joyce R, Johnson A . Iatrogenic vCJD from surgical instruments. BMJ 2001; 322: 1558–1559
Collinge J . Variant Creutzfeldt-Jakob disease. Lancet 1999; 354: 317–323
Bernoulli C, Siegfried J, Baumgartner G, Regli F et al. Danger of accidental person-to-person transmission of Creutzfeldt–Jakob disease by surgery. Lancet 1977; 1: 478–479
Advisory committee on dangerous pathogens and spongiform encephalopathy advisory committee. Transmissible Spongiform Encephalopathy Agents: Safe Working and the Prevention of Infection Stationery Office: London 1998
Lueck CJ, McIlwaine GG, Zeidler M . Creutzfeldt–Jakob disease and the eye. I. Background and patient management. Eye 2000; 14: 263–290
Beran RF . Cleaning of ophthalmic diamond scalpels. J Refract Corneal Surg 1994; 10: 582–586
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented as a poster at the annual congress of Royal College of Ophthalmologists at Birmingham, in May 2001.
Rights and permissions
About this article
Cite this article
Dinakaran, S., Kayarkar, V. Debris on processed ophthalmic instruments: a cause for concern. Eye 16, 281–284 (2002). https://doi.org/10.1038/sj.eye.6700132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6700132
Keywords
This article is cited by
-
Fibres found in the eye during and after phacoemulsification cataract surgery
Eye (2014)
-
Pre-sterilisation cleaning of re-usable instruments in general dental practice
British Dental Journal (2007)
-
A model for the management of an atypical endophthalmitis outbreak
Eye (2005)
-
Debris on processed ophthalmic instruments: a cause for concern
Eye (2003)
-
Reply: Debris on instruments
Eye (2003)