Abstract
Objective: The clinical efficacy, tolerability and acceptability of a new multidose powder inhaler (MDPI) [Easyhaler®, Orion Pharma, Finland] containing a high dose (500 μg/dose) of beclomethasone dipropionate (BDP) were compared with those of BDP metered dose inhaler administered with a large volume spacer (MDI-spacer).
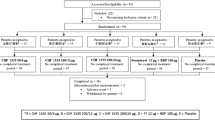
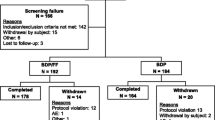
Patients and Study Design: Recruited patients were adult asthmatics currently receiving 800 to 1000 μg/day of inhaled corticosteroid. The dose of BDP during the study was 1000 μg/day The study was an open, randomised, parallel-group multicentre study and included a 2-week run-in period followed by a 12-week treatment period.
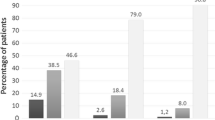
Results: 74 patients were randomised to both groups. During the run-in period the mean morning peak expiratory flow (PEF) was 489 and 478 L/min in the MDPI and MDI-spacer groups, respectively. During the last 2 weeks of the study the morning PEF was 485 L/min in the MDPI group and 477 L/min in the MDI-spacer group. Asthma symptom scores and use of rescue medication were low in both groups. The median dose of histamine required to decrease forced expiratory volume in 1 second (FEV1) by 15% was 1.05mg in the MDPI group and 0.64mg in the MDI-spacer group. The most frequent adverse events were hoarseness and sore throat. Mean serum cortisol levels were not affected in either treatment group. Patients’ personal opinion regarding acceptability of the devices clearly favoured the MDPI.
Conclusion: In conclusion, the novel powder inhaler was well tolerated and at least equally effective compared with the conventional MDI-spacer combination in the treatment of asthma with BDP. However, in everyday use the patients clearly favoured the powder inhaler.
Similar content being viewed by others
References
National Heart, Lung, and Blood Institute, National Institute of Health. International consensus report on diagnosis and treatment of asthma. Eur Respir J 1992; 5: 601–41
Hurter T. Asthmabronchiale und antiasthmatika. Deutsche Apoth Zeit 1987; 127: 837–41
Newman SP, Miller AB, Lennard-Jones TR, et al. Improvement of pressurized aerosol deposition with Nebuhaler spacer device. Thorax 1984; 39: 935–41
Crompton GK. Clinical use of dry powder systems. Eur J Respir Dis 1982; 63: 96–9
Lindgren S, Bake B, Larsson S. Clinical consequences of inadequate inhalation technique in asthma therapy. Eur J Respir Dis 1987; 70: 93–8
Berglund E. Inhalation therapy problems and prospects. Eur Respir J 1990; 3: 830–2
Yarbrough J, Lyndon RN, Mansfield E, et al. Metered dose inhaler induced bronchospasm in asthmatic patients. Ann Allergy 1985; 55: 25–7
Crompton GK. New inhalation devices. Eur Respir J 1988; 1: 679–80
Viljanen AA, Halttunen PK, Kreus K-E, et al. Spirometric studies in non-smoking, healthy adults. Scand J Clin Lab Invest 1982; 42Suppl. 159: 5–20
American Thoracic Society. Medical Section of the American Lung Association Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1987; 136: 225–44
Sovijärvi A, Malmberg P, Reinikainen K, et al. Arapid dosimetric method with controlled tidal breathing for histamine challenge. Chest 1993; 104: 164–70
Shaw NJ, Edmunds AT. Inhaled beclomethasone and oral candidiasis. Arch Dis Child 1986; 61: 788–90
Barnes NC, Marone G, Di Maria GU, et al. A comparison of fluticasone propionate 1mg daily, with beclomethasone dipropionate, 2mg daily, in the treatment of severe asthma. Eur Respir J 1993; 6: 877–84
Ebden P, Jenkins A, Houston G, et al. Comparison of two high dose corticosteroid aerosol treatments, beclomethasone dipropionate (1500 μg/day) and budesonide (1600 μg/day), for chronic asthma. Thorax 1986; 41: 869–74
Brown PH, Blundell G, Greening AP, et al. Hypothalamo-pituitary-adrenal axis suppression in asthmatics inhaling high dose corticosteroids. Respir Med 1991; 85: 501–10
Farrer M, Francis AJ, Pearce SJ. Morning serum Cortisol concentrations after 2mg inhaled beclomethasone dipropionate in normal subjects: effect of a 750ml spacing device. Thorax 1990; 45: 740–2
Brown PH, Blundell G, Greening AP, et al. Do large volume spacer devices reduce the systemic effects of high dose inhaled corticosteroids? Thorax 1990; 45: 736–9
Newman SP, Millar AB, Lennard-Jones TR, et al. Improvement of pressurised aerosol deposition with Nebuhaler spacer device. Thorax 1984; 39: 935–41
Newman SP, Moren F, Pavia D, et al. Deposition of pressurized suspension aerosols inhaled through extension devices. Am Rev Respir Dis 1981; 124: 317–20
Selroos O, Halme M. Effect of a volumatic spacer and mouth rinsing on systemic absorption of inhaled corticosteroids from a metered dose inhaler and dry powder inhaler. Thorax 1991; 46: 891–4
Haahtela T, Vidgren M, Nyberg A, et al. A novel multidose powder inhaler. Salbutamol powder and aerosol give equal bronchodilatation with equal doses. Ann Allergy 1994; 72: 178–82
Nieminen MM, Vidgren M, Laurikainen K, et al. Easyhaler®, a novel multidose powder inhaler: clinically equivalent to salbutamol metered dose inhaler and easier to use. Respiration 1994; 61: 37–41
Vidgren M, Silvasti M, Vidgren P, et al. Easyhaler® multidose powder inhaler — practical and effective alternative to the pressurized MDL Aerosol Sci Technol 1995; 22: 335–45
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poukkula, A., Alanko, K., Kilpiö, K. et al. Comparison of a Multidose Powder Inhaler Containing Beclomethasone Dipropionate (BDP) with a BDP Metered Dose Inhaler with Spacer in the Treatment of Asthmatic Patients. Clin. Drug Investig. 16, 101–110 (1998). https://doi.org/10.2165/00044011-199816020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199816020-00002