Article Text
Abstract
Background Worldwide, the emergence of super-ageing societies has increased the number of older people requiring support for daily activities. Many elderly residents of nursing homes (NHs) take drugs to treat chronic conditions; however, there are few reports of medication safety in NHs, especially from non-western countries.
Objective We examined the incidence and nature of adverse drug events (ADEs) and medication errors (MEs) in NHs for the elderly in Japan.
Design, setting, and participants The Japan Adverse Drug Events Study for NHs is a prospective cohort study that was conducted among all residents, except for short-term admissions, at four NHs for older people in Japan for 1 year.
Measurements Trained physicians and psychologists, five and six in number, respectively, reviewed all charts of the residents to identify suspected ADEs and MEs, which were then classified by the physicians into ADEs, potential ADEs and other MEs after the exclusion of ineligible events, for the assessment of their severity and preventability. The kappa score for presence of an ADE and preventability were 0.89 and 0.79, respectively.
Results We enrolled 459 residents, and this yielded 3315 resident-months of observation time. We identified 1207 ADEs and 600 MEs (incidence: 36.4 and 18.1 per 100 resident-months, respectively) during the study period. About one-third of ADEs were preventable, and MEs were most frequently observed in the monitoring stage (72%, 433/600), with 71% of the MEs occurring due to inadequate observation following the physician’s prescription.
Conclusion In Japan, ADEs and MEs are common among elderly residents of NHs. The assessment and appropriate adjustment of medication preadmission and postadmission to NHs are needed to improve medication safety, especially when a single physician is responsible for prescribing most medications for the residents, as is usually the case in Japan.
- Medication safety
- Adverse events, epidemiology and detection
- Medical error, measurement/epidemiology
- Nursing homes
- Pharmacoepidemiology
Data availability statement
Data are available on reasonable request. On reasonable request, derived data supporting the findings of this study are available from the corresponding author (Nobutaka Ayani, E-mail: lingren@koto.kpu-m.ac.jp, ORCID ID:0000-0003-1130-052x) after approval from the institutional review board of the Kyoto Prefectural University of Medicine.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- Medication safety
- Adverse events, epidemiology and detection
- Medical error, measurement/epidemiology
- Nursing homes
- Pharmacoepidemiology
Key messages
What is already known on this topic
Few reports are available on medication safety in nursing homes (NHs) for older people, and the reports from non-western countries are rather limited in terms of the sample sizes and outcomes.
What this study adds
Since the accumulation of region-specific data is essential for improving quality of care, we determined the incidence, nature and potential risk factors for adverse drug events (ADEs) and medication errors (MEs) in NHs for older people in Japan, one of the world’s largest aging countries.
How this study might affect research, practice and/or policy
Clarification of the actual epidemiology of ADEs and MEs in Japanese NHs would be beneficial for extrapolation to other countries that also have aging populations.
Introduction
Population ageing is an emerging issue worldwide. In 2020, the percentage of older people (age ≥65 years) in developed countries was 16.6% in the USA, 18.1% in Canada, 18.7% in the UK, 20.8% in France, 21.7% in Germany and 23.3% in Italy, whereas the rate in Japan was 28.4% (about 36 million) and constitutes the highest proportion of the elderly population worldwide.1 With an ageing society, the number of older people who need support for daily activities also increases. From 2000 to 2018, the number of people aged 65 and above who received long-term care services increased approximately three times (from 1.8 million to 5.5 million, ie, from 8% to 16% of all elderly aged ≥65 years) in Japan.2
Consequently, the number of older people availing residential care services, such as nursing homes (NHs), has been increasing annually. The number of elderly residential care users in 2018 was 2.1 million, an increase of approximately 2.6 times compared with 0.8 million in 2000, accounting for approximately 38% of all elderly service users in Japan.3 This trend has also been observed in other developed countries. In the USA, approximately 5.7 million people (12% of 47 million people aged ≥65 years) used long-term care services in 2016, of which 33% (approximately 1.9 million) received residential care services.4 In the future, many countries will witness an increase in the number of elderly residential care users due to the growing demand for elderly care services.
Many older people receive pharmacotherapy for chronic conditions. In a previous study, 20% of older people in the USA had five or more chronic conditions, and 50% of this population were taking five or more medications.5 Furthermore, 40% of elderly NH residents receive nine or more medications in the USA.6 Many elderly residential care users are at risk for problems related to medication use, such as adverse drug events (ADEs) and medication errors (MEs). In particular, they often experience dementia,7 8 and psychotropic drugs used to treat their psychiatric symptoms can cause a variety of health problems.9–13 However, few reports are available on medication safety in NHs,14–17 and the reports from non-western countries are rather limited in terms of the sample sizes and outcomes.16 17 The quality of medical and nursing care varies greatly by region; thus, accumulation of region-specific data is essential for improving quality of care. Japan is among the nations with the largest ageing population in the world, and clarification of the actual epidemiology of ADEs and MEs in NHs in Japan would be beneficial for extrapolation to other countries that also have ageing populations. The purpose of this study was to examine the incidence, nature and potential risk factors of ADEs and MEs in NHs for older people in Japan.
Methods
Study design and participants
The Japan Adverse Drug Events (JADE) study comprises a series of multicentre cohort studies conducted in several clinical settings.18–20 As part of the JADE study series, this prospective cohort study was conducted for older people in Japan who require daily support. Long-term care facilities (LTCFs) for the elderly in Japan are classified into three and four types of public and private facilities, respectively, depending on the level of care and length of stay.2 Of these, all private facilities and about 8% of public facilities are classified as assisted living facilities, while about 4% of public facilities are classified as sanatoriums; therefore, 88% of public facilities (46% of all facilities) are classified as NHs.2 In NH, most prescriptions were made by a single commissioned physician. This study was conducted in four NHs (280 beds in total).
Data were collected from all residents, except for short-term admissions that mainly concerned family respite for a few days, from 1 August 2016 to 31 July 2017, regardless of whether the resident was admitted or discharged during the period. This study was approved by the institutional review board (IRB) of the authors’ university and was performed in accordance with the principles underlying the Declaration of Helsinki.21 Since data were collected as part of the daily clinical practice, informed consent was replaced by the opt-out method, where IRB-approved explanatory documents were posted to residents and their families during the study period.
Definitions
The primary outcome was ADEs, defined as drug-related injuries resulting from medical intervention.22 23 ADEs comprise multiple components, ranging from harm caused by drugs used at a usual dosage (adverse drug reactions) or an unusual dosage, to harm from dose reduction and discontinuation of medication.23 For example, an extrapyramidal symptom, such as parkinsonism, that occurs after a patient receives antipsychotics and without any other apparent cause, is considered an ADE, as is rebound insomnia that occurs following the discontinuation of sedatives.
An ADE was categorised by severity as fatal, life-threatening, serious or significant,24 based on whether it resulted in death; caused life-threatening issues such as respiratory depression or severe hypotension; induced moderate symptoms, such as falls with harm, anuria or minor bleeding (eg, gastrointestinal bleeding) and resulted in milder cases, including diarrhoea, constipation, drowsiness, fluid leakage into tissues or falls without harm, respectively.
The secondary outcome was MEs, which can occur at any stage in the medication use process (ordering by physicians; transcribing by nurses; dispensing by pharmacists; storing by nurses/caregivers; administration by nurses/caregivers and monitoring by physicians/nurses/caregivers) and may or may not cause ADEs.24 MEs were classified by the type of error (misprescription (incorrect name/dose/route/time, forgetting prescription, prescribing prohibited drugs), transcription errors, dispensing errors (faulty equipment, wrong drug distribution), administrative errors (incorrect name/dose/route/time, forgetting administration), misplaced drugs and inadequate observation after administration), the stage in the process where it occurred, and the job title most responsible for its occurrence (physicians, nurses, pharmacists, caregivers, residents and their family, among others). ADEs were categorised as either preventable or non-preventable. An ADE was considered preventable if it resulted from an ME. For example, an antibiotic-induced rash in patients with no previous drug-induced rash would not be considered a preventable ADE, but it would be considered so (ADE with administrative error) in patients with a history of such a rash. In another example, the first fall that occurs due to antipsychotic drug administration to patients with severe agitation would not be considered a preventable ADE, whereas repeated falls due to continued administration of the same prescription would be assessed as such (ADEs with monitoring errors).
Furthermore, we classified ADEs according to their injury-causing potential. A potential ADE was an ME that had the potential for but did not actually result in injury, either because of specific circumstances or chance, or because the ME was intercepted.24 For example, administration several hours earlier of hypnotics that caused no negative effects would be considered a potential ADE because hypnotics may cause immediate somnolence. In contrast, administration at noon of antidementia drugs, such as donepezil, that should be administered in the morning would be classified as an ME but not a potential ADE, because this scenario rarely causes any harm.
Data collection and classification
The definitions and methods used in this study are consistent with those reported in previous related studies.24 In a previous study that explored the reasons for NH placement, approximately half were attributed to behavioural problems associated with dementia;7 another study revealed that 37.8%–88.6% of the elderly care facility residents have dementia.8 Therefore, in this research, five psychiatrists and six clinical psychologists with sufficient knowledge and experience in care of elderly patients with dementia divided the chart review of all care records, along with records pertaining to prescriptions, laboratories and incident reports, under the supervision of two internists with sufficient experience on this topic.18 19 25 26 All reviewers were trained in chart review based on the reported methods.24 All psychiatrists had 6 years of medical education at university, followed by a 2-year residency in various departments including internal medicine according to the Japanese medical education programme and two of the psychiatrists had three to 6 years of clinical experience as internists before becoming psychiatrists. In addition, although Japanese clinical psychologists are not licensed to prescribe, the psychologists involved in this study had some knowledge of medications used for the elderly through their clinical experience and they could be consulted by the accompanying psychiatrists directly or by the psychiatrists over the phone during the chart review.
Prior to the chart review, the reviewers collected information on the characteristics of residents in the cohort either at the outset of the study or when new residents were admitted during the study period. After collecting this information, ADEs and MEs were identified by chart review. When suspected ADEs or MEs were identified during chart review, reviewers recorded the event details, including medication information (generic name, dose, route and class). Symptoms were recorded for suspected ADEs; type, stage and persons-in-charge when the error occurred were recorded for suspected MEs. The comorbidity of the participants was quantified using the Charlson Comorbidity Index (CCI),27 and functional independence was evaluated using the Barthel Index (BI).28 The presence of dementia was assessed based on whether a diagnosis of dementia was recorded in the case record or antidementia medication was prescribed.
After details of the suspected events were collected, the physicians independently classified relevant events as an ADE, a potential ADE and other MEs, or they excluded the event. Moreover, all incidents were classified according to their causative drug class (including psychotropic or not), severity and preventability. The association between an ADE and the drug was determined according to the Naranjo algorithm29 as well as published reports that showed an association between a particular medication and an ADE. After the independent review, all physicians confirmed the final classification for each incident. When the physicians disagreed on the classification of an event, a consensus was reached through discussion.
Statistical analyses
The unit of observation was ‘resident-months’ (of the NH stay) for calculating the incidence. The incidence per 100 resident-months and 95% CIs were calculated. Continuous variables are presented as means with SDs or medians with IQRs, and categorical variables are presented as numbers and percentages. We used Fisher’s exact test to test for differences in ADE severity due to drug initiation, continuation or dose increase and dose reduction or discontinuation.
We used multivariable logistic regression models to assess the relationships between ADEs or MEs or preventable ADE and potential risk factors. The unit of observation was ‘resident’ for logistic regression, and the dependent variable was ‘occurrence of ADEs, MEs and preventable ADEs’. The models included older age (≥85 years), sex (female), dementia (absent), number of medications used until the start of the study (≥5), number of psychotropic drugs used until the start of the study (≥1), CCI (≥3) and BI (totally independent (≥85), minimally dependent (≥60, <85), partially dependent (≥40, <60) and very dependent (<40)). We only examined resident-related risk factors and included these factors simultaneously without model selection.
To assess inter-reviewer agreement, Cohen’s kappa was calculated from the results of an independent review by two psychiatrists, using random sampling of 120 events from 1600 suspicious events collected in the first stage of the review. The kappa score between reviewers regarding the presence of an ADE was 0.89 (95% CI 0.79 to 0.98) (ADE vs ME without ADE or exclusion). The kappa for preventability was 0.79 (95% CI 0.67 to 0.90) (preventable vs non-preventable), whereas that for severity was 0.65 (95% CI 0.51 to 0.79) (significant vs serious or life-threatening or fatal). These values are similar to those reported from previous studies by Gurwitz15 and Morimoto18 that used the same methodology. P<0.05 were considered statistically significant. All analyses were performed using JMP V.14.0 software (SAS Institute, Cary, North Carolina, USA).
Results
Overall, this study included 459 residents, with 3315 resident-months (median 7.6 months (IQR 2.6–12); mean 7.2 months (SD 4.5)) during the study period. At the outset, 271 residents were already admitted to the facilities, and 188 new residents were admitted during the study period. Of all residents, 42% (193/459) were discharged during the study period, while 27% discharges (52/193) were due to death. The mean age was 86.6 (SD 6.9) years; 64% (293/459) of the residents were aged ≥85 years and 75% (344/459) were female. The median CCI was 1 (IQR 1–3), and approximately 32% (146/459) of residents had CCI≥3. The median BI was 50 (IQR 25–80), and the evaluation of functional independence showed that 22% (100/459), 25% (113/459), 22% (100/459) and 32% (146/459) residents were totally independent, minimally dependent, partially independent and very dependent, respectively. The median number of medications used until study commencement was 4 (IQR 2–6), and 47% of the residents received five or more medications (table 1).
Demographic data of the study population
Adverse drug events
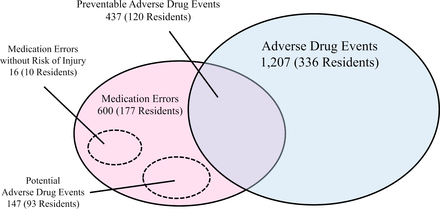
We identified 1600 suspected incidents, and through reviews and discussions about them, we identified 1207 ADEs among 336 residents (73%; figure 1). The incidence of ADEs was 36.4 (95% CI 34.4 to 38.5) per 100 resident-months. The incidence of fatal, life-threatening and serious ADEs was 0.3 (95% CI 0.1 to 0.5), 1.1 (95% CI 0.7 to 1.4) and 4.6 (95% CI 3.9 to 5.3), respectively. Regarding severity of ADEs, 30% (3/10) of fatal ADEs and 43% (15/35) of life-threatening ADEs were due to psychotropic drugs. Approximately 5% (60/1207) of all ADEs were caused by drug dose reduction or discontinuation. Of them, 8.3% (5/60) were fatal or life-threatening ADEs, which was higher than the percentage of ADEs due to drug initiation, continuation, or dose increase (3.5%, 40/1147); however, the difference was not statistically significant (p=0.067). When stratified by type of ADEs, neuropsychiatric symptoms were the most frequent, accounting for 46% (559/1207) of all ADEs, followed by gastrointestinal symptoms (26%, 310/1207) and cardiovascular symptoms (11%, 132/1207). In terms of individual symptoms, falls (40%, 480/1207) were the most frequent (table 2).
Relationship between adverse drug events and medication errors.
Severity and type of adverse drug events
The most common drug class associated with ADEs included sedatives (benzodiazepine receptor agonists (BZDRAs); 15.7%, 189/1207) and atypical antipsychotics (15.7%, 189/1207), followed by antihypertensives (9.9%, 120/1207), laxatives (9.5%, 115/1207), mood stabilisers (8.4%, 101/1207) and fluids and electrolytes (7.0%, 84/1207). Approximately 60% of ADEs (688/1207) were associated with psychotropic drugs (table 3).
Frequency of adverse drug events and medication errors according to each drug class
Several factors were associated with ADEs in the multivariate analysis (table 4). Participants who received five or more medications, those who received one or more psychotropic drugs, participants with dementia and those who were partially functionally dependent also had a higher risk for ADEs.
Factors associated with adverse drug events, medication errors and preventable adverse drug events
Medication errors and potential adverse events
We identified 600 MEs in 177 residents (39%; figure 1), with an incidence of 18.1 (95% CI 16.7 to 19.5) per 100 resident-months. Among the 600 MEs, 437 (73%) resulted in ADEs; thus, one-third of all ADEs were preventable ADEs. In contrast, 147 of the MEs had the potential to cause injury but did not result in observable harm (figure 1). The incidence of preventable and potential ADEs was 13.2 (95% CI 11.9 to 14.4) and 4.4 (95% CI 3.7 to 5.2) per 100 resident-months, respectively. Only 16 MEs carried no risk of injury to participants; 8% of potential ADEs (12 cases) were intercepted before a drug was administered and were thus classified as intercepted potential ADEs.
MEs were most frequently associated with the monitoring stage (72%, 433/600 in 118 residents), followed by the administering (19%, 115/600 in 66 residents), ordering (8%, 47/600 in 19 residents), dispensing (0.5%, 3/600 in 3 residents) and storing (0.3%, 2/600 in 3 residents) stages. Among preventable ADEs, the high frequency of the monitoring stage was more evident (89%, 387/437), followed by the ordering (10%, 42/437), administering (1%, 6/437), dispensing (0.2%, 1/437) and storing (0.2%, 1/437) stages. Physicians were the most common occupation responsible for the occurrence of ME (59%, 353/600), followed by nurses (24%, 140/600), caregivers (16%, 98/600) and others (2%, 9/600). The majority of MEs that physicians and nurses were responsible for were observed in the monitoring stage (87%, 306/353; 91%, 127/140, respectively), with 71% of all MEs occurring due to inadequate observation following the physician’s prescription. Typical physician’s ME involving continued prescription of the same drug that caused ADEs without adequate evaluation, and a typical nurse’s ME comprising interruption of medication due to fluid leakage or self-removal of the intravenous tube by the resident.
Factors associated with MEs included older age, receiving five or more medications and participants who were partially or very functionally dependent; factors associated with preventable ADEs, in addition to aforementioned factors, included dementia and receiving one or more psychotropic drugs—except for older age (table 4).
Discussion
We determined that ADEs and MEs were common in the Japanese NH setting. ADEs and MEs were observed in 73% and 39% of the participants, with an incidence of 36 and 18 per 100 resident-months, respectively. Approximately one-third of the ADEs were preventable. We identified that all MEs and preventable ADEs occurred most frequently at the monitoring stage (72% and 89%, respectively).
Compared with two previous studies,14 15 which were conducted in elderly residential care settings using the same chart-review methodology, the incidence of ADEs, potential ADEs and preventable ADEs in this study (36.4 ADEs, 4.4 potential ADEs and 13.2 preventable ADEs per 100 resident-months) was much higher than that in NHs (1.9 ADEs and 0.7 potential ADEs per 100 resident-months)14 and LTCFs (9.8 ADEs and 4.1 preventable ADEs per 100 resident-months)15 in the USA. However, a thorough comparison of the results revealed several contradictions related to the much higher incidence in this study than in the previous studies. First, the resident background (age, sex and duration of stay) in this study (87±7 years, 75% and 7.2 months, respectively) and the previous studies (84±9 years, 77% and 9.9 months in NHs; 86±8 years, 72% and 6.7 months in LTCFs, respectively)14 15 was similar. In addition, the estimated number of drugs per resident, based on the data described in the previous studies, was 4.9 in NHs14 and 7.7 in LTCFs,15 which were similar to or greater than the mean number of drugs (4.6) that residents received in this study. Third, regarding causative drugs, 57% of ADEs were caused by psychotropic drugs in this study, which was generally similar to the results of the previous studies, where psychotropic drugs accounted for 61% of ADEs in NHs and 39% in LTCFs.
In contrast to these points, when stratified by the severity of ADEs (fatal and life-threatening vs serious vs significant), this study had a higher number of ADEs classified as significant ADEs (45 vs 153 vs 1009) compared with the previous studies (32 vs 206 vs 308 in NHs; 37 vs 188 vs 590 in LTCFs).14 15 The difference with regard to severity of ADEs may be because the care records in this study were comprehensive and physicians with sufficient experience in clinical practice and clinical epidemiology were deeply involved in the chart-review stage, which resulted in the collection of many events of lesser severity. Regarding MEs, the definition and estimation of previous studies and this study differed slightly. If we assume that several types of MEs not assessed in the previous studies occur in the same proportions as those in this study, the incidence of MEs would be 2.9 in NHs and 8.6 in LTCFs per 100 resident-months.14 15 In addition, monitoring errors mainly comprised insufficient postmedication examination in the previous studies, and the high incidence of MEs in this study may be because we also considered continued prescription without adequate evaluation of the drug-caused ADEs and inadequate observation during intravenous infusion. A study of MEs in elderly care facilities in the UK, using more detailed methodology, showed that the proportion of residents with MEs was higher than that in this study (70%, 178/256 vs 39%, 177/600), except for monitoring errors (11%, 27/256 vs 20%, 118/600).30 Thus, we believe that this study’s results, which revealed more ADEs and MEs compared with the previous studies, may more accurately represent the incidence of ADEs and MEs in elderly residential care settings.
Previous reports have highlighted the risk of polypharmacy31 32 and psychotropic drugs14 15 in ADEs—corresponding with our results. In contrast, this study also showed a noteworthy result that ADEs due to dose reduction or discontinuation tend to be more fatal and life-threatening than other components (8.3% vs 3.5%), although they are not significantly different (p=0.067). In Japan, patients are prescribed medications by multiple specialists separately until they are admitted to a facility; however, most medications are prescribed by a single commissioned physician after admission, which may result in ADEs due to unfamiliar medication adjustment. Therefore, to improve medication safety in elderly residential care settings, residents should ideally receive intervention from general practitioners and pharmacists for the appropriate adjustment of the multiple medications they are taking preadmission and postadmission.
Regarding the risk of MEs and preventable ADEs, partially and very dependent residents had an approximately twofold increased risk. With long-term medication use, postmedication follow-up is likely to be neglected, thus repeatedly resulting in ADEs with the same medication. Therefore, this study’s results suggest that careful monitoring of medication use is imperative in NHs to avoid preventable ADEs, especially for residents who require a high level of care.
Our study has some limitations. First, this study was conducted in four facilities classified as NHs, which account for about half of the LTCFs for the elderly in Japan; however, these facilities are located in a limited area, and residents of assisted living facilities and sanatoriums were not included. Further studies are needed to clarify the epidemiology of ADEs and MEs among residents of LTCFs for the entire elderly population in Japan. Second, some events that had not been described in the care records could not be evaluated, implying that our results underestimated the true incidence. However, more robust alternatives for measuring ADEs and MEs are yet to be developed, and the approach that we used is the most common. Third, the descriptions in the care records about physicians and pharmacists work were relatively scarce compared to those about nurses and caregivers work; this may have affected the paucity of ordering and dispensing errors. However, previous studies on long-term residents or long-term inpatients showed a similar composition of errors as in this study;14 15 20 therefore, the composition of the MEs in this study is considered reasonable. Fourth, psychotropic drugs may have been frequently detected as causative drugs of the incident because this study was mainly conducted by psychiatrists. However, the proportion of psychotropic drugs as causative drugs was also high in a previous study in an elderly residential care setting in the USA, even though primary care physicians and pharmacists were primarily involved instead of psychiatrists.14 15 In addition, two of the psychiatrists who participated in this on-site chart review were well experienced in internal medicine, and this study was conducted with sufficient support from two internists who were familiar with clinical epidemiological studies. Therefore, we believe that the classification of causative drugs may not be significantly biased by the characteristics of the researchers. Finally, our study evaluated only the most suspicious single drug and did not evaluate the dosage of the drugs and drug interactions in drug coadministration. Many residents receive various types of medications for a long period, and it is difficult to assess how the total dosage of multiple medications could affect incidents. Thus, further studies are needed to identify the effect of the total amount of multiple medications on ADEs and MEs to determine desirable medication adjustments that could reduce ADEs and MEs in the future.
Conclusion
ADEs and MEs occur frequently in four Japanese NHs for older people. Although polypharmacy may be associated with a high frequency of ADEs and MEs, the deprescription may also carry a risk of severe ADEs. Therefore, caution in drug adjustment and monitoring is imperative. Appropriate assessment of prescriptions by general practitioners and pharmacists, comparison of medication regimens preadmission and postadmission and the development of a care system customised according to the characteristics of the residents, such as the presence or absence of dementia and level of care, are required to reduce the risk of ADEs and MEs.
Data availability statement
Data are available on reasonable request. On reasonable request, derived data supporting the findings of this study are available from the corresponding author (Nobutaka Ayani, E-mail: lingren@koto.kpu-m.ac.jp, ORCID ID:0000-0003-1130-052x) after approval from the institutional review board of the Kyoto Prefectural University of Medicine.
Ethics statements
Patient consent for publication
Acknowledgments
We are indebted to Ms. Kumi Ohta, Ms. Mayuri Matsuda, Mr. Sho Yoneda, Ms. Haruka Okamoto, Ms. Kaori Tachi, Ms. Misa Fujii, Ms. Masami Hiyama, and all the staff of the cooperative institution for their assistance and generosity with this project. We presented portions of an earlier version of this manuscript at the International Society for Quality in Healthcare’s 35th and 37th International Conferences that took place in 2018 and 2021.
References
Footnotes
Contributors All authors were involved in the design of the study. NA, NO, RK, AK and JN collected the data. NA analysed the data under the technical supervision of TM and MS. NA and TM interpreted study results, and NA wrote the first draft of the manuscript. All authors reviewed the manuscript, provided substantive intellectual contributions and approved the final version of the manuscript for publication. NA is responsible for the overall content as guarantor.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.